Abstract
Objective
To investigate the significance of the dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) parameters of diffuse spinal bone marrow infiltration in patients with hematological malignancies.
Materials and Methods
Dynamic gadolinium-enhanced MR imaging of the lumbar spine was performed in 26 patients with histologically proven diffuse bone marrow infiltration, including multiple myeloma (n = 6), acute lymphoblastic leukemia (n = 6), acute myeloid leukemia (n = 5), chronic myeloid leukemia (n = 7), and non-Hodgkin lymphoma (n = 2). Twenty subjects whose spinal MRI was normal, made up the control group. Peak enhancement percentage (Emax), enhancement slope (ES), and time to peak (TTP) were determined from a time-intensity curve (TIC) of lumbar vertebral bone marrow. A comparison between baseline and follow-up MR images and its histological correlation were evaluated in 10 patients. The infiltration grade of hematopoietic marrow with plasma cells was evaluated by a histological assessment of bone marrow.
Results
Differences in Emax, ES, and TTP values between the control group and the patients with diffuse bone marrow infiltration were significant (t = -11.51, -9.81 and 3.91, respectively, p < 0.01). Emax, ES, and TTP values were significantly different between bone marrow infiltration groups Grade 1 and Grade 2 (Z = -2.72, -2.24 and -2.89 respectively, p < 0.05). Emax, ES and TTP values were not significantly different between bone marrow infiltration groups Grade 2 and Grade 3 (Z = -1.57, -1.82 and -1.58 respectively, p > 0.05). A positive correlation was found between Emax, ES values and the histological grade of bone marrow infiltration (r = 0.86 and 0.84 respectively, p < 0.01). A negative correlation was found between the TTP values and bone marrow infiltration histological grade (r = -0.54, p < 0.01). A decrease in the Emax and ES values was observed with increased TTP values after treatment in all of the 10 patients who responded to treatment (t = -7.92, -4.55, and 5.12, respectively, p < 0.01).
Patients with hematological malignancies such as multiple myeloma, lymphoma, and leukemia, frequently undergo the replacement of spinal hemopoietic bone marrow. Focal nodular bone marrow involvement of the spine in malignant diseases and the use of contrast media has been the subject of previous magnetic resonance (MR) studies (1-3). However, in early diffuse bone marrow infiltration, tumor cells do not displace enough bone marrow fat cells, hence the amount remains relatively normal. The appearance of spinal bone marrow on T1- and T2-weighted spin-echo MR images is often normal in patients with early diffuse bone marrow invasion by hematological malignancies and is even normal in up to one-quarter of patients with stage III multiple myeloma (3).
Highly cellular hematopoietic marrow cannot be distinguished reliably from highly cellular neoplastic infiltration by using routine gadopentetate enhanced scans. Recently, dynamic contrast-enhanced MR imaging (DCE-MRI) based on time-intensity curves (TIC), which depicts the normal physiological features of the microcirculation, has been successfully used in the patients with lymphoproliferative diseases, diffuse bone marrow infiltration, and in the assessment of response to chemotherapy (4-9). However, there have been few studies evaluating DCE-MRI parameters in cases of spinal bone marrow infiltration (6). Thus, the purpose of this study was to investigate the value of DCE-MRI parameters of diffuse spinal bone marrow infiltration in patients with hematological malignancies.
The study was approved by our Institutional Review Board. Subjects were enrolled from a 558-patient database that received a routine spinal MRI examination in our hospital from April 2007 to August 2008. All subjects gave written informed consent before any examination.
According to this database, we prospectively enrolled the only 26 consecutive patients, among the 558 patients, who fulfilled the following criteria: (a) histologically proven bone marrow infiltration and (b) no focal or combined focal and diffuse bone marrow abnormalities at MR imaging.
Among the 26 patients, six had multiple myeloma (6 males, age range: 56-75 years, mean age: 68.3 years), six patients had acute lymphoblastic leukemia (2 females, 4 males, age range: 15-79 years, mean age: 44.8 years), five patients had acute myeloid leukemia (3 females, 2 males, age range: 12-69 years, mean age: 49.0 years), seven patients had chronic myeloid leukemia (3 females, 4 males, age range: 12-66 years, mean age: 45.5 years), and two patients had diffuse large B-cells non-Hodgkin lymphoma (NHL) (2 males, 25 years and 41 years, respectively).
Twenty subjects (5 females, 15 males, age range: 41-61 years, mean age: 52.1 years) whose spinal MRI was normal, made up the control group. These healthy subjects did not have bone marrow disease, underlying malignancy, or a recent episode of trauma.
The routine MRI sequences and dynamic study for 26 patients and 12 healthy subjects were performed within a 1-hour interval.
An MRI examination of the thoracolumbar spine area was performed approximately 1-3 days prior to the bone marrow biopsy. All of the patients underwent MR imaging with a 1.5-Tesla superconducting system and Synergy spine coil (Gyroscan Intera, Philips Medical System, The Netherlands). The MR imaging protocol was identical for all of the patients: T1-weighted spin-echo sequences with a TR of 480 msec, TE of 11 msec, and T2-weighted turbo spin-echo sequences with a TR of 3000 msec, and a TE of 120 msec. These sequences were performed with the following parameters: 256×512 matrix, 33-cm field of view, and sagittal 4-mm-thickness contiguous sections with an interval of 0.4 mm.
Dynamic studies of gadolinium enhancement were performed with a T1-weighted turbo field echo (TFE) sequence using following parameters: 6.4/3.8 (repetition time msec/echo time msec), a 30° flip angle, a 224×224 matrix, an acquisition time of 1,000 msec, a 35-cm field of view, and a midsagittal, 10-mm section thickness. A bolus of gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany; 0.1 mmol/kg body weight) was rapidly injected with a power injector at a rate of approximately 3 ml/s using a 21-gauge intravenous catheter through the right antecubital vein. This was followed by a 20-ml saline flush at the same injection rate. The dynamic imaging was initiated 5 seconds before injection of the contrast medium. In total, 100 dynamic images were obtained within 100 seconds (0.98 second per frame) in each patient. The post-contrast T1-weighted spin-echo sequence was begun 3-5 minutes after the end of the bolus injection of gadoterate meglumine.
A post treatment DCE-MRI was performed in 12 patients; one with multiple myeloma, four with acute lymphoblastic leukemia, three with acute myeloid leukemia, and four with chronic myeloid leukemia after an interval of 4-6 months.
Bone marrow signal intensity was analyzed qualitatively by consensus between two radiologists. By comparing the signal intensity of the bone marrow with the signal intensity of adjacent muscle tissue depicted on sagittal T1-weighted spin-echo MR images, two radiologists defined the change in signal intensity in terms of low signal intensity (i.e., lower than the signal intensity of muscle), iso-signal intensity (i.e., similar to the signal intensity of muscle) and high signal intensity (i.e., higher than the signal intensity of muscle).
Signal intensity (SI) values were measured in operator-defined regions of interest (ROIs) in the same vertebral body (mostly L3 or L4) for perfusion analysis. The regions of interest were placed centrally and anteriorly in the vertebral body to avoid the end plates, possible disk degeneration, and to avoid the basivertebral venous plexus and cerebrospinal fluid.
The SI values derived from the ROIs were plotted against time as a TIC using the Gyroview software package (Gyroscan Intera, Philips Medical Systems, The Netherlands). The baseline value for signal intensity (SIbase) in a TIC was defined as the mean SI from the first three images. The maximum signal intensity (SImax) was defined as the peak enhancement value for the first pass of contrast injection. The contrast rise time (Trise) was defined as the rise time interval from the time point of SIbase to that of SImax. The contrast time to peak (TTP) enhancement was defined as the time point of SImax.
The peak enhancement percentage of (Emax) of the lumbar vertebral and enhancement slope (ES) was used as an index for lumbar vertebral bone marrow perfusion. These perfusion indices were calculated as:
The above analysis was assessed quantitatively by one radiologist.
The bone marrow biopsies were obtained by a standard technique (trephine, aspirate or a combination of both) from the posterior iliac crest. The biopsies were dehydrated and embedded in methyl methacrylate without decalcification. Next, semi thin sections of 2µm were obtained and subsequently stained with Hematoxylin and Eosin (HE). Bone marrow infiltration was graded according to the percentage of neoplastic cells in the bone marrow samples (Vol%): Grade 0, less than 5% of the non-fat cells were occupied by tumor cells; Grade 1, 5-25% of the nonfat cells were occupied by tumor cells; Grade 2, 25-75% of the non-fat cells were occupied by tumor cells; and Grade 3, more than 75% of the non-fat cells were occupied by tumor cells. Grades 0 and 1 were considered to represent none or mild tumor infiltration, at complete or near complete remission, respectively. Whereas, Grades 2 and 3 were considered to be intermediate and extensive tumor infiltration, respectively.
Descriptive statistical data were expressed as the means ± standard deviation. An independent sample t-test was performed to analyze the statistical significance of perfusion parameters between the infiltration and control group. The Mann-Whitney U test was performed to analyze the statistical significance of perfusion parameters among different histological grading. A Spearman coefficient (r) correlation was performed between infiltration degree and perfusion parameters. A paired sample t-test was performed to analyze the statistical significance of perfusion parameters at pre- and post-chemotherapy treatment MR imaging. A two-sided p value of 0.05 or less was considered statistically significant. The various statistical testing was performed using the SPSS 10.0 software package (SPSS Inc., Chicago, IL).
Bone marrow biopsies showed a Grade 1 infiltration in three patients with acute lymphoblastic leukemia and three patients with acute myeloid leukemia, a Grade 2 infiltration in one patient with acute lymphoblastic leukemia, two patients with acute myeloid leukemia, two patients with chronic myeloid leukemia, and one patient with multiple myeloma, a Grade 3 infiltration in two patients with acute lymphoblastic leukemia, five patients with chronic myeloid leukemia, five patients with multiple myeloma, and two patients with diffuse large B-cells non-Hodgkin lymphoma.
No obvious abnormality was observed in the lumbar bone marrow on the sagittal T1-weighted spin-echo images of three patients with acute lymphoblastic leukemia and three patients with acute myeloid leukemia (Fig. 1). A sagittal T1-weighted spin-echo image revealed diffuse homogeneous low signal intensity in three patients with acute lymphoblastic leukemia, two patients with acute myeloid leukemia, seven patients with chronic myeloid leukemia, one patient with multiple myeloma, and two patients with diffuse large B-cell non-Hodgkin lymphoma (Fig. 2). A heterogeneous signal with mixed areas of low and high signal intensity, described as a pepper and salt sign, was found in five patients with multiple myeloma.
The differences in Emax, ES, and TTP values between the control group and the patients with diffuse bone marrow infiltration were significant (t = -11.51, -9.81 and 3.91, respectively, p < 0.01). The mean Emax, ES and TTP values were 260% (± 31.3%), 11% (± 2.8%), and 24.67 (± 2.99) for the malignant infiltration group, 94% (± 64%), 3% (± 2.6%), and 33.44 (± 10.96) for the normal control group, respectively (Fig. 3). Differences in Emax, ES, and TTP values between the Grade 1 and Grade 2 infiltration group were significant (Z = -2.72, -2.24 and -2.89, respectively, p < 0.05). The differences in the Emax, ES, and TTP values between the Grade 1 and Grade 3 infiltration group were also significant (Z = -2.72, -2.97 and -2.85, respectively, p < 0.01). However, the differences in Emax, ES, and TTP values between the Grade 2 and Grade 3 infiltration group were not significant (Z = -1.57, -1.82 and -1.58, respectively, p > 0.05) (Table 1). A positive correlation was found between the Emax, ES values and the bone marrow infiltration histological grade of, respectively (r = 0.86 and 0.84, respectively, p < 0.01). A negative correlation was found between the TTP values and the histological grade of bone marrow infiltration (r = -0.54, p < 0.01).
A comparison of the signal intensities of the vertebral bone marrow before and after the application of contrast agent revealed no significant differences for the sagittal Gadolinium-enhanced T1-weighted spin-echo sequence in the control group. Compared to pre-enhanced T1-weighted imaging, post-enhanced T1-weighted imaging showed no obvious enhancement of vertebral bone marrow in six patients, diffuse homogeneous enhancement in 15 patients, and inhomogeneous enhancement in five patients.
During follow-up observation, bone marrow biopsies were not performed in one patient with multiple myeloma and one patient with chronic myeloid leukemia. A decrease in the Emax and ES values with a corresponding increase in the TTP values was observed after treatment in the other 10 patients who positively responded to the treatment (t = -7.92, -4.55 and 5.12, respectively, p < 0.01) (Table 2). Gadolinium-enhanced sagittal T1-weighted imaging showed a pronounced reduction of contrast enhancement after therapy in those patients. The corresponding change in the post treatment histologic grades improved from Grade 3 to Grade 1 in all 10 patients.
For hematological malignancies, the diffuse infiltration of bone marrow typically occurs in axial skeleton, such as skull, spine, sternum, and pelvis. It is difficult to distinguish diffuse tumor infiltration from the highly variable appearance of normal bone marrow, due to the effects of age and sex for routine MRI sequences. Various MR imaging techniques have been developed to improve the detection and quantification of diffuse bone marrow infiltration. These techniques include chemical-shift imaging, fat suppression imaging, bulk T1 relaxation time measurement, and proton MR spectroscopy. However, these quantitative methods have failed to help in improving the MR imaging-based detection of diffuse bone marrow infiltration (6).
Progression of hematological malignancies is accompanied by an increase in bone marrow neovascularization, owing to the increased angiogenic potential of bone marrow tumor cells. Evidence for an increase in angiogenesis in hematological malignancies originated from the expression of angiogenic cytokines, infiltration of metalloproteinases, and an increase in blood vessel density in the angiogenic process (8). In contrast to the histological detection of microvessel density, DCE-MRI has the advantage of being functionally variable and providing the opportunity for subsequent examinations including a much larger area of investigation (10). DCE-MRI is based up on an open two-compartment model and has become increasingly important for estimating tissue physiological parameters such as perfusion, capillary permeability, and the relative volume of extravascular extracellular space. The Emax has been regarded as a complex process, including blood inflow, outflow, transit, and permeability factors that affect extracellular compartment gadolinium concentration. This parameter indicates the concentration of contrast material in the extracellular space of the vertebral bone marrow (11). The enhancement slope is mainly determined on the basis of tissue vascularization; that is, the number of vessels, the degree of perfusion, and capillary permeability. DCE-MRI has been used to correlate dynamic MRI features with prognostic factors in solid tumor, such as breast cancer (12). Recent studies have shown that DCE-MRI can distinguish between normal and malignant spinal bone marrow infiltration (4, 6). Furthermore, DCE-MRI may identify malignant bone marrow infiltration in patients with negative static MRI and serve both as a diagnostic and prognostic tool for patients with spinal bone marrow malignancies (7). Other data also demonstrated that DCE-MRI provided independent evidence for angiogenesis in multiple myelomas (8). Our results showed that differences in Emax, ES and TTP values between the control group and the patients with diffuse bone marrow infiltration were significant, which implied that DCE-MRI parameters can reflect the perfusion of spinal bone marrow infiltration and demonstrate the grades of diffuse bone marrow infiltration in hematological malignancies.
It is important to identify bone marrow infiltration grades in patients with hematological malignancies for evaluating the clinical staging and treatment response. A previous study has reported that significant differences in Emax, slope, and washout were found between subjects with normal bone marrow and patients with diffuse bone marrow infiltration. Emax, slope, and washout values increased with increasing bone marrow infiltration grade (6). Our results indicated that a positive correlation was found between Emax, ES values, and histological grade of bone marrow infiltration. Meanwhile, a negative correlation was found between the TTP values and histological grade of diffuse bone marrow infiltration. Thus, DCE-MRI may be helpful in the diagnosis and grading of diffuse spinal bone marrow infiltration. However the differences in Emax, ES and TTP values between patients in infiltration groups Grade 2 and Grade 3 infiltration group were not significant. We presume that an increasing contribution and the exudation of gadopentetate dimeglumine in extracellular space are mainly due to an increase in bone marrow microvessel permeability and decreased membrane integrity in patients with Grade 2 and Grade 3 bone marrow infiltration. Consequently, compared to patients a Grade 1, an extracellular contrast agent is more rapidly cleared from the intravascular space, and the equilibrium in the extracellular space is attained more rapidly for patients with both Grade 2 and Grade 3 bone marrow infiltration. In addition, our results are preliminary since the number of patients with Grade 1 or Grade 2 is relatively small. Further study of tumor angiogenesis in the specimens is recommended to clarify this issue.
The initial results of a clinical histological study involving patients with multiple myelomas revealed that after chemotherapy, the mean microvessel density was significantly reduced in responders compared with non-responders (9, 10). As mentioned above, our results also showed a decrease in the Emax and ES values with a corresponding increase in the TTP values after treatment of patients with hematological malignancies among patients responding well to treatment. This finding implies that DCE-MRI could be used to monitor bone marrow perfusion in the follow-up of patients receiving antiangiogenic therapy.
This study had several limitations. First, the histological specimen in the case studies was obtained at the posterior iliac crest and not in the vertebral body, which implies that the sites of the biopsies and MRI measurements did not match. It is not practical to perform routinely vertebral biopsies in order to identify the histological bone marrow infiltration grades in these patients. So far, there has been no evidence of anatomic differences for diffuse infiltration between the spinal and iliac crest bone marrow in patients with hematological malignancies. We believe that iliac bone marrow biopsies are still an accurate and sensitive method for evaluating the tumor cells burden of bone marrow. Second, although our preliminary study results show decreased Emax, ES values and corresponding increased TTP values after treatment in 10 patients with hematological malignancies who responded well to treatment, we acknowledge that the number of patients followed up with MR imaging were limited. Third, a previous study showed Emax and wash-out values of normal spinal bone marrow were significantly higher in patients younger than 40 years than in those aged 40 years or older (13). In patients younger than 40 years, spinal bone marrow consists of bulk hematopoietic red marrow with a rich vessel network. Further studies will be needed in order to define the real difference in Emax and ES values between Grade 1 infiltration and the normal spinal bone marrow in subjects younger than 40 years.
In conclusion, dynamic contrast-enhanced MR imaging of the spine can demonstrate diffuse bone marrow infiltration of hematological malignancies, whereas the DCE-MRI parameters can reflect histological grade of bone marrow infiltration.
Figures and Tables
Fig. 1
25-year-old man with acute lymphoblastic leukemia.
A. Precontrast T1-weighted spin-echo image of lumbar spine shows no signal intensity changes, however dynamic contrast-enhanced MRI perfusion imaging of TFE-T1 weighted image obtained at 20 sec, 40 sec and 60 sec after gadopentetate dimeglumine bolus injection shows enhancement of vertebral bone marrow (Emax = 256.12%, ES = 10.68% and TTP = 20 sec).
B. L4 vertebral body of same patient shows TIC with initial rapidly rising slope, followed by second slow rising phase.
C. Bone marrow biopsy image of same patient with acute lymphoblastic leukemia shows severe tumor cell infiltration (Hematoxylin & Eosin staining, ×200).
D. Decreased Emax and ES values with increased TTP values (Emax = 192.56%, ES = 7.56% and TTP = 25 sec) were observed after treatment in same patient who responded well to treatment.
TFE = turbo field echo, Emax = peak enhancement percentage, ES = enhancement slope, TTP = time to peak, TIC = time-intensity curve
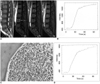
Fig. 2
50-year-old patient with acute myeloid leukemia.
A. Pre-contrast T1-weighted spin-echo image of lumbar spine shows diffuse low signal intensity consistent with bone marrow infiltration. Dynamic contrast-enhanced MRI perfusion imaging of TFE-T1 weighted image obtained at 20 sec, 40 sec and 60 sec after gadopentetate dimeglumine bolus injection shows enhancement in vertebral bone marrow (Emax = 225.58%, ES = 10.74% and TTP = 22 sec).
B. L3 vertebral body of same patient shows TIC with rapidly rising slope (wash-in) during initial short period.
C. Bone marrow biopsy image of same patient (Fig. 3) with acute myeloid leukemia shows moderate tumor cell infiltration (Hematoxylin & Eosin staining, ×400).
D. Decreased Emax and ES values with increased TTP values (Emax = 135.35%, ES = 5.12% and TTP = 26.5 sec) were observed after treatment in same patient who responded well to treatment.
TFE = turbo field echo, Emax = peak enhancement percentage, ES = enhancement slope, TTP = time to peak, TIC = time-intensity curve
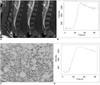
Fig. 3
35-year-old man in normal control group.
A. Dynamic contrast-enhanced MRI perfusion imaging of TFE-T1 weighted image obtained at 10 sec, 30 sec and 50 sec after gadopentetate dimeglumine bolus injection shows enhancement in vertebral bone marrow (Emax = 116.42%, ES = 4.67% and TTP = 30.5 sec).
B. Vertebral body of L4 of same patient shows TIC with initial slowly rising slope followed by platform phase.
TFE = turbo field echo, Emax = peak enhancement percentage, ES = enhancement slope, TTP = time to peak, TIC = time-intensity curve

References
1. Stäbler A, Baur A, Bartl R, Munker R, Lamerz R, Reiser MF. Contrast enhancement and quantitative signal analysis in MR imaging of multiple myeloma: assessment of focal and diffuse growth patterns in marrow correlated with biopsies and survival rates. AJR Am J Roentgenol. 1996. 167:1029–1036.
2. Vande Berg BC, Lecouvet FE, Michaux L, Ferrant A, Maldague B, Malghem J. Magnetic resonance imaging of the bone marrow in hematological malignancies. Eur Radiol. 1998. 8:1335–1344.
3. Lecouvet FE, Vande Berg BC, Michaux L, Malghem J, Maldague BE, Jamart J, et al. Stage III multiple myeloma: clinical and prognostic value of spinal bone marrow MR imaging. Radiology. 1998. 209:653–660.
4. Zhang L, Mandel C, Yang ZY, Yang Q, Nibbs R, Westerman D, et al. Tumor infiltration of bone marrow in patients with hematological malignancies: dynamic contrast-enhanced magnetic resonance imaging. Chin Med J. 2006. 119:1256–1262.
5. Nosàs-Garcia S, Moehler T, Wasser K, Kiessling F, Bartl R, Zuna I, et al. Dynamic contrast-enhanced MRI for assessing the disease activity of multiple myeloma: a comparative study with histology and clinical markers. J Magn Reson Imaging. 2005. 22:154–162.
6. Rahmouni A, Montazel JL, Divine M, Lepage E, Belhadj K, Gaulard P, et al. Bone marrow with diffuse tumor infiltration in patients with lymphoproliferative diseases: dynamic gadoliniumenhanced MR imaging. Radiology. 2003. 229:710–717.
7. Moulopoulos LA, Maris TG, Papanikolaou N, Panagi G, Vlahos L, Dimopoulos MA. Detection of malignant bone marrow involvement with dynamic contrast-enhanced magnetic resonance imaging. Ann Oncol. 2003. 14:152–158.
8. Moehler TM, Hawighorst H, Neben K, Egerer G, Hillengass J, Max R, et al. Bone marrow microcirculation analysis in multiple myeloma by contrast-enhanced dynamic magnetic resonance imaging. Int J Cancer. 2001. 93:862–868.
9. Baur A, Bartl R, Pellengahr C, Baltin V, Reiser M. Neovascularization of bone marrow in patients with diffuse multiple myeloma: a correlative study of magnetic resonance imaging and histopathologic findings. Cancer. 2004. 101:2599–2604.
10. Hillengass J, Wasser K, Delorme S, Kiessling F, Zechmann C, Benner A, et al. Lumbar bone marrow microcirculation measurements from dynamic contrast-enhanced magnetic resonance imaging is a predictor of event-free survival in progressive multiple myeloma. Clin Cancer Res. 2007. 13:475–481.
11. Chen WT, Ting-Fang Shih T, Hu CJ, Chen RC, Tu HY. Relationship between vertebral bone marrow blood perfusion and common carotid intima-media thickness in aging adults. J Magn Reson Imaging. 2004. 20:811–816.
12. Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008. 9:10–18.
13. Montazel JL, Divine M, Lepage E, Kobeiter H, Breil S, Rahmouni A. Normal spinal bone marrow in adults: dynamic gadolinium-enhanced MR imaging. Radiology. 2003. 229:703–709.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download