Abstract
Objective
To evaluate our early experience using self-expanding stents to treat atherosclerotic vertebral artery ostial stenosis (VAOS), with respect to technical feasibility and clinical and imaging follow-up results.
Materials and Methods
A total of 20 lesions in 20 patients underwent stenting of the VAOS using a self-expanding stent (Precise RX; Cordis Neurovascular, Miami Lakes, FL). Two patients were asymptomatic. We analyzed the technical success rate, causes of technical failure, occurrence of any vascular or neurological event, and the occurrence of any neurological abnormality or in-stent restenosis (ISR) seen on follow-up. The imaging follow-up was performed with Doppler ultrasound (DUS) as a primary screening modality.
Results
One instance of technical failure was caused by failure of the guidewire passage. The stent diameter was 5 mm, and post-stenting balloon dilatations were necessary in all cases. Stent misplacement requiring placement of an additional stent occurred in four cases. Following a 14.8 month average clinical follow-up time, two patients showed anterior circulation ischemia, which was not attributed to the VAOS we treated. Following a 13.7 month average DUS follow-up, five patients showed a mild degree of diffuse or focal intimal thickening in the stent lumen; however, none of the stenosis showed luminal loss of more than 50% and no stent fracture was noted.
Atherosclerotic stenosis of the vertebral artery (VA) at the ostium is not a rare clinical condition and has been considered clinically benign (1). With the frequent use of CT angiography (CTA) or MR angiography (MRA), an increasing number of lesions have recently been discovered. According to the New England Medical Center Posterior Circulation Registry, contrary to the generally accepted notion, approximately one-third of the patients with posterior circulation ischemia had VA ostial stenosis (VAOS) (2, 3). It is generally believed that the main mechanism of ischemia with VAOS is artery-to-artery embolism (2-5).
Vertebral artery ostial stenosis has been treated medically and intervention has been indicated only in selected patients. Surgery, such as VA-common carotid artery transposition, was performed on very rare occasions until it was replaced by endovascular techniques such as a balloon angioplasty or stent replacement (6-12).
As was already reported by the CAVATAS (carotid and vertebral artery transluminal angioplasty study) trial, angioplasty and/or stenting of VAOS was feasible but showed a high restenosis rate. Several case-series studies have reported higher technical success rates (95-100%) with relatively high in-stent restenosis (ISR) rates (20-50%) seen on follow-up (7-10, 13). Although symptomatic ISR is relatively rare, its occurrence rate can be disappointing, thus making it tempting to use drug-eluting stents (14, 15). With the drug-eluting stents, the ISR rate has decreased significantly; however, the procedures were not free from occasional stent fractures which were already seen with balloon-expandable stents (BES) in VAOS (16, 17).
As had already been suggested by Weber et al. (12), we also felt the need for a new technical approach for treating this particular lesion. Due to our continuing accumulation of experience with self-expanding carotid stents, we assumed that the physical properties of a self-expanding stent (SES), such as its longitudinal, radial, and rotational flexibility and its continuous radial expansile force, might help to adjust to the peculiar anatomical environment of a VAOS.
Therefore, the purpose of our report is to analyze our experience with SES for the endovascular treatment of atherosclerotic VAOS with respect to technical feasibility, immediate clinical and angiographic outcomes, and clinical and imaging follow-up outcomes.
From our neurointerventional patient database, we extracted a total of 20 patients who had undergone stent-assisted revascularization of VAOS or proximal VA stenosis from December 2003 to April 2008. All patients were men whose average age was 64.3 years, with a range of 47 to 74 years. Among these patients, nine presented with an acute stroke in the posterior circulation, whereas the nine others presented with symptoms of vertebrobasilar insufficiency without any notable brain parenchymal lesion seen on the imaging studies. Lesions were incidentally discovered during vascular evaluation of coexisting internal carotid artery disease in two patients. Diagnosis of the VA stenosis was initially assessed by MRA, and the lesion characteristics were further evaluated by way of a digital subtraction angiography (DSA) in all patients. The site of stenosis was at the ostium in 17 patients and in the proximal VA before entering the transverse foramen without involvement of the ostium in three patients. The mean diameter of the target vessel in the distal sound segment was 3.9 (2.9-5.2) mm. The mean degree of stenosis before the procedure was 73% (56-91%). The mean length of stenosis was 7.7 (3-25) mm. Indications for stent implantation included symptomatic stenosis with more than 50%, or asymptomatic lesion with significant stenosis of the dominant VA in patient with bilateral stenoses, or asymptomatic unilateral significant stenosis with a- or hypo-plastic contralateral VA which includes a small VA with a posterior inferior cerebellar artery ending. The significant stenosis was defined as more than 70% of stenosis. The degree of stenosis was calculated using following formula: [1 - (diameter of stenotic segment / distal vessel diameter avoiding poststenotic dilatation)] × 100 (%).
For the elective procedures, the patients were pretreated with aspirin (100 mg daily following a 500- or 300-mg loading dose) and clopidogrel (75 mg daily following a 300-mg loading dose) for 2-5 days before the procedure. In an acute setting, the loading doses of clopidogrel and aspirin were administered via a nasogastric tube immediately before the procedure or stent implantation. Informed consent was obtained from each patient or relative. Under local anesthesia, the patient was anticoagulated with intravenous heparin after obtaining diagnostic angiograms of both the cerebral arteries and the stenotic VA. The heparin dose was adjusted to achieve an ACT (activated clotting time) of 2-2.5 times that of the baseline. A 6-Fr long sheath (COOK Shuttle Bloomington, IN) or an 8-Fr guiding catheter (Envoy; Cordis Neurovascular, Miami Lakes, FL) was introduced into the proximal subclavian artery. If the position of the tip of the guiding catheter was unstable, insertion of an additional sturdy guidewire (Glidewire; Boston Scientific Corp., Fremont, CA) as a 'buddy' wire far into the ipsilateral brachial artery was helpful for the maintenance of guiding catheter stability during the procedure. The lesion was crossed using a microguidewire (Transend Floppy 300; Boston Scientific Corp.). In some cases, it was necessary to use a microcatheter due to the difficulty of the lesion cross. The lesion was predilated using a 2.0-3.0-mm (Maverick; Boston Scientific Corp.) or 4-mm-diameter (Ultrasoft; Boston Scientific Corp.) balloon angioplasty catheter. After ballooning, a self-expanding stent (Precise RX; Cordis Neurovascular) was deployed (Fig. 1). We attempted to minimize redundancy of the proximal segment of the stent within the subclavian artery; however, we were not always successful. Post-dilation was performed using a 4- or 5-mm-diamater angioplasty balloon catheter (Ultrasoft; Boston Scientific Corp.). The placement of an anti-embolic distal protection device was not part of our routine procedure.
After the procedure, the patient was transferred to the neuro-intensive care unit for overnight observation in the event of any vascular or nonvascular adverse reaction. Patients were educated and medicated in order to, as much as possible, correct any modifiable vascular risk factors. Clinical and radiological follow-ups were scheduled at 3, 6, and 12 months after the procedure and annually thereafter. For the imaging follow-up, we performed a duplex ultrasound to evaluate stent patency due to its availability and non-invasiveness. DSA was reserved for the patients who showed significant ISR on Doppler ultrasound (DUS).
We analyzed the technical success rate, which was defined as the successful delivery of the stent to the target lesion without significant residual stenosis. The cause of technical failure was identified. We found that the most difficult step of the procedure was the exact deployment of the stent along with sufficient covering of the lesion. We analyzed the rate of stent misplacement which required another stent placement. The degree of residual stenosis was calculated again immediately after the procedure. We analyzed the occurrence of acute in-stent thrombosis or distal embolization as well as any vascular or neurological event. The clinical and radiologic follow-up results were analyzed for the occurrence of any neurological abnormality or ISR. Significant ISR was defined as a greater than 50% diameter narrowing. Since there is a lack of solid DUS criteria for the significant ISR, we relied mostly on the presence and degree of intimal thickening on the gray scale images. We graded the degree of intimal thickening as mild (less than 30% of luminal loss), moderate (less than 50% of luminal loss) and severe (more than 50% of luminal loss). Patterns of the intimal thickening were classified as diffuse or focal and peak systolic flow velocities were measured from the multiple points in the stented segment and the highest value was recorded to use as an ancillary finding in the estimation of the significance of the lumen loss. Stent mesh integrity was evaluated with the DUS.
The technical success rate for stent placement was 95%. One case of technical failure occurred as a result of a failure in the guidewire passage through the stenosis. Data on each patient were summarized in the Table 1, which includes the summary of the data from the 19 patients who underwent successful stent placement. Stent diameter was 5 mm in most cases, and the length of the stent ranged from 20 to 50 mm. A second stent was required in four patients because of the misplacement of the first stent. The main cause of misplacement was distal jumping of the stent, thereby failing to cover the most stenotic portion of the lesion, which was at the os. Post-balloon dilation was required in all cases because of the significant residual stenosis, even after stent placement. After post-dilation, there was no significant residual stenosis in all cases, leaving a mean residual stenosis rate of 7% and a range of 0-25%. There was no case of acute in-stent thrombosis at the completion of the procedure. Furthermore, there was no case of any neurologic abnormality after the procedure. Two patients showed unexplained blood pressure fluctuation immediately after the procedure; however, they were stabilized without any subsequent clinical consequences.
A final clinical and imaging follow-up was obtained at an average of 14.8 (range, 3.2-40.9) months and 13.7 (range, 3.1 to 39.2) months after the procedure, respectively. Two of the 19 patients showed neurological abnormalities during the follow-up period. However, their symptoms were not attributed to the VAOS that was treated. In one patient who was suffering from underlying lymphoma, recurrent infarction in the anterior circulation territory was present, and there were recurrent transient ischemic attacks in the carotid artery territory in the other patient who had metastatic disease of an unknown primary origin. On follow-up DUS, the stented segment, was successfully visualized in all cases and showed mild degree (less than 30% of luminal loss) of intimal hyperplasia. The pattern of stenosis was diffuse in three cases and focal in two cases. None of the lesions showed significant ISR. Further, on Doppler examination, the mean of the highest peak systolic velocity measured within the stented segment was 99.5 (46-260) cm/sec. As the patient who showed a peak systolic velocity of 260 cm/sec did not show any luminal loss on gray scale images, this could be explained as compensatory increase of the flow velocity because the stented VA was the only patent supraaortic artery in this particular patient. DUS demonstrated placed stents which remained intact without deformity or fracture.
Like other ostial lesions, such as those in renal or coronary arteries, previous reports indicate that VAOS has a high risk of ISR. The reported ISR rates with bare-metal BESs are between 35 and 45% (7, 8, 12, 13, 18, 19), which is approximately 10-15% greater than the rate with bare metal stenting in the coronary arteries and much greater than that after carotid stenting. This is consistent with the stenting results seen in coronary ostial lesions, which show almost two-fold higher ISR rates than usual non-ostial lesions (20, 21, 36). Considering those study results, our follow-up study showed the best long-term patency rate and durability. The only difference between our report and those reports was the type of stent used for the revascularization (i.e. SES versus BES).
There have been several possible explanations of frequent ISR in VAOS after BES placement, all of which attempted to determine the reason based on the peculiar anatomic characteristics of the lesion. As seen in other arterial ostia, the one in VA also has a well-developed, thick tunica media resembling the features of the adjacent subclavian artery. Therefore, an atherosclerotic lesion in that region may have a substantial plaque burden because the lesion is part of the atherosclerotic process involving the subclavian artery (11). This would basically explain the disappointing long-term results of conventional balloon angioplasty and BESs in the treatment of VAOS. In addition, the tightness of an ostial lesion might make it refractory to balloon angioplasty due to the frequent elastic recoil or residual stenosis, even with a stent, because of the abundance of muscularis and plaque in the region (7).
Some researchers have emphasized the importance of the type or the material from which a stent is made. Taylor et al. (19) reported that the use of a non-cobalt chromium type of stent, such as a stainless steel stent, showed a significantly higher rate of ISR on follow-up. They hypothesized that the thinner struts in the cobalt-chromium type of stents could reduce ISR.
Another factor to be considered is the continuous mobility of the subclavian and VA junction, which could cause a stent to be bent or flexed during motion. The difference in flexibility between the artery and the stent might cause continuous irritation of the stented segment and eventually provoke an excessive intimal response to the stent or even fracture of the stent itself (22). Chronic metallic fatigue of the relatively rigid BES, together with the endless mobility of the region, has seemed to be the cause of occasional stent fracture, which in turn could be a cause of early loss of stent patency (17).
Some researchers have approached this situation in a different way. They have suggested that the hemodynamic complexity of the acutely angled junction between the larger subclavian artery and the smaller VA can provoke change of wall shear, which is believed to be important for the excessive intimal hyperplasia and aggravation of atherosclerosis in the stented segment (7, 23, 24).
To overcome the frequent ISR in VAOS, there have been several reports regarding the application of drug-eluting stents. With only limited cases, as we have seen in coronary ostial lesions (21), significant improvement of the ISR rate as low as 0-7% has been reported (14, 25). However, those drug treated stents were basically of the balloon expandable type and were hence not free from occasional fractures (16).
We believe that the excellent follow-up results with our cases can be explained by the superior performance of the SES (Precise RX, Cordis Neurovascular) we used. This stent is an open-cell type and made of laser-cut nitinol (26, 27). The potential superiority of SESs over BESs may be related to the difference in their mechanical properties. The more radial and longitudinal flexibility of the SES may reveal its superior conformability to the peculiar anatomy in the VAOS. However, we cannot tell which of the stent's characteristics has played the primarily beneficial role as there could be several possible explanations. Because of the sufficient radial expansile force of the SES, there is probably little chance of elastic recoil once the lesion is fully dilated after a post-stenting balloon angioplasty, which we performed in every patient. Continuous radial force might promote the long term patency of the SES as Wakhloo et al. (28) observed in their experimental study. Regarding the continuous mobility of the region, the longitudinal flexibility of the nitinol stent is probably more adaptable than the rigid, balloon-expandable stent, which has little flexibility (29). This characteristic might also explain the absence of stent fractures in our series.
Regarding the diameter of the target vessels, the currently available self-expanding vascular stents, the smallest being 5 mm in diameter, were not suitable for VAOS, since the mean vessel diameter in our series was 3.9 mm. However, owing to the latitude of the selfexpanding nature of the stent in a smaller-diameter artery, there was no incidence of over-expansion since we used 4-mm-diameter balloon catheters for post-dilation in smaller arteries. We believe that obtaining a vessel diameter greater than 4 mm might help to reduce the ISR, as was reported in renal ostial stenting cases (24). Nevertheless, we felt that we also needed smaller diameter stents, e.g. 4.0 or 4.5 mm. In addition, shorter stents, such as a 1.5-cm length, would help avoid unnecessary stent placement crossing the C6 transverse foramen.
From a technical viewpoint, differing from the stenting technique using BESs, placement of SESs can be difficult because of their poor controllability. As Wehman et al. (11) clearly described in their review article, exact positioning of even a BES to cover an entire lesion, while minimizing the protrusion of the free struts into the subclavian artery lumen, could be challenging since the VAOS was usually focal and very tight. To achieve this goal with an SES is more challenging, which is why we initially experienced frequent stent misplacements, which required the use of an additional stent.
The following procedures might help to reduce misplacement of stents (11, 30, 31): (a) Stable positioning of a guiding catheter with use of a buddy wire if more stability is required; (b) Not relying on roadmap images for lesion localization during the stent positioning as lesions are mobile. The most reliable technique for lesion visualization is intermittent flushing of the guiding catheter with contrast medium; (c) Patient breath-hold during unsheathing of the stent delivery system. General anesthesia should be considered if the patient is unable to cooperate during the procedure. In addition, shortening the stent after deployment should also be considered, although this phenomenon occurs minimally with laser-cut stents; (d) Stop unsheathing if there is any flaring of the distal stent markers. The patient should have the freedom to breath and recheck the position of the stent by a brief flushing with contrast media since this will be the last opportunity for repositioning the stent. Next, the patient should perform another breath-hold and unsheathe the entire stent with no hesitation; (e) If there is a possibility of misplacement, another option is to place the stent, while leaving a sufficient proximal free segment in the subclavian artery lumen, as we usually did for additional stent placement. We did not observe any specific problems when using this technique.
Another technical consideration could be the use of distal protection devices. Actually, we used Filterwire (Boston Scientific Corp.) in two cases with seemingly very large plaque burdens. There was not much difficulty in the placement of the device or in stent deployment; however, there was a substantial amount of difficulty in post-dilation and retrieval of the protection device since the proximal end of the stent protruded into the subclavian artery lumen where the arterial course was deflected. The proximal struts of the deployed stent protruding into the subclavian artery lumen served as an obstacle to passing catheters through the stent lumen. After this experience, the use of a distal protection device was no longer part of our routine. Although, Divani et al. (32) observed the same frequency and number of captured emboli between stenting in carotid stenosis and in VAOS, there has not generally been much concern regarding the distal embolism in VAOS unlike in carotid stenosis.
Since the occurrence of bilateral VAOS stenoses are quite common, the choice of a target lesion can be an issue. Considering the relative benign clinical consequence of VAOS, we chose symptomatic lesion or dominant VA lesion as our treatment target in the case of bilateral VAOS stenoses. However, bilateral stenting could be done if one cannot tell which lesion was symptomatic or the diameters of the two VAs were the same.
There are some limitations to our study. First, it was performed retrospectively and with a relatively small number of patients. The clinical and imaging follow-up periods varied despite our planned periodic follow-up schedule. We also lacked a conventional angiographic correlation in evaluating stent patency during follow-up, which may be crucial for the accurate evaluation of ISR.
As far as vascular imaging follow-up is concerned, we believe DUS can serve as an effective screening tool for ISR after stenting of VAOS since the role of CTA is still limited (33, 34). We are not suggesting that DUS can replace DSA in the evaluation of ISR; however, the technique was able to determine whether or not there was significant ISR. Although it has been reported that visualization of V1 segment with DUS could be unsuccessful occasionally (35), we could examine the stented segment without much difficulty in all cases since the implanted stent played as a good anatomic landmark for the clear visualization of the V1. However, we suggest that a correlation study with DSA be performed in order to validate the accuracy of DUS.
In conclusion, the use of self-expanding stents for the treatment of the VAOS is technically feasible and may help to improve the long-term patency of the artery during our limited follow-up interval. However, size limitation of the currently available stent diameter and occasional misplacement of the stent requiring placement of an additional stent remained as technical barriers.
Figures and Tables
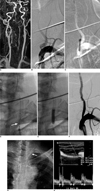
Fig. 1
Placement of self-expanding stent in 68-year-old man who presented two weeks before procedure with right side weakness and ataxia due to multifocal embolic lesions in posterior circulation.
A. Contrast-enhanced MR angiography shows bilateral ostial stenoses. Left vertebral artery ostial stenosis lesion was considered to be cause.
B. Digital subtraction angiography shows focal severe stenosis of ostium.
C. Lesion is pre-dilated with 4-mm angioplasty balloon.
D. Self-expanding stent (Cordis Precise RX, 5 mm × 20 mm) is placed over stenotic lesion, which leaves residual stenosis (arrow).
E. Residual stenosis is successfully dilated using 5-mm balloon angioplasty catheter.
F. Final control angiogram shows minimal residual stenosis without flow restriction.
G. Chest radiograph obtained 12 months after procedure shows full expansion of whole stent.
H. Clinical and Doppler ultrasound follow-up performed 21 months after procedure shows good patency of stent without significant intimal hyperplasia.
References
1. Fisher CM. Occlusion of the vertebral arteries. Causing transient basilar symptoms. Arch Neurol. 1970. 22:13–19.
2. Wityk RJ, Chang HM, Rosengart A, Han WC, DeWitt LD, Pessin MS, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998. 55:470–478.
3. Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, et al. New England Medical Center Posterior Circulation Registry. Ann Neurol. 2004. 56:389–398.
4. Koroshetz WJ, Ropper AH. Artery-to-artery embolism causing stroke in the posterior circulation. Neurology. 1987. 37:292–295.
5. Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal PA, Belkin M, et al. Embolism from vertebral artery origin occlusive disease. Neurology. 1992. 42:1505–1512.
6. Piotin M, Spelle L, Martin JB, Weill A, Rancurel G, Ross IB, et al. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am J Neuroradiol. 2000. 21:727–731.
7. Albuquerque FC, Fiorella D, Han P, Spetzler RF, McDougall CG. A reappraisal of angioplasty and stenting for the treatment of vertebral origin stenosis. Neurosurgery. 2003. 53:607–614.
8. SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004. 35:1388–1392.
9. Ko YG, Park S, Kim JY, Min PK, Choi EY, Jung JH, et al. Percutaneous interventional treatment of extracranial vertebral artery stenosis with coronary stents. Yonsei Med J. 2004. 45:629–634.
10. Lin YH, Juang JM, Jeng JS, Yip PK, Kao HL. Symptomatic ostial vertebral artery stenosis treated with tubular coronary stents: clinical results and restenosis analysis. J Endovasc Ther. 2004. 11:719–726.
11. Wehman JC, Hanel RA, Guidot CA, Guterman LR, Hopkins LN. Atherosclerotic occlusive extracranial vertebral artery disease: indications for intervention, endovascular techniques, short-term and long-term results. J Interv Cardiol. 2004. 17:219–232.
12. Weber W, Mayer TE, Henkes H, Kis B, Hamann GF, Holtmannspoetter M, et al. Efficacy of stent angioplasty for symptomatic stenoses of the proximal vertebral artery. Eur J Radiol. 2005. 56:240–247.
13. Coward LJ, McCabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007. 38:1526–1530.
14. Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, et al. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 2006. 37:2562–2566.
15. Qureshi AI, Kirmani JF, Harris-Lane P, Divani AA, Ahmed S, Ebrihimi A, et al. Vertebral artery origin stent placement with distal protection: technical and clinical results. AJNR Am J Neuroradiol. 2006. 27:1140–1145.
16. Kim SR, Baik MW, Yoo SH, Park IS, Kim SD, Kim MC. Stent fracture and restenosis after placement of a drug-eluting device in the vertebral artery origin and treatment with the stent-instent technique. Report of two cases. J Neurosurg. 2007. 106:907–911.
17. Tsutsumi M, Kazekawa K, Onizuka M, Kodama T, Matsubara S, Aikawa H, et al. Stent fracture in revascularization for symptomatic ostial vertebral artery stenosis. Neuroradiology. 2007. 49:253–257.
18. Chastain HD 2nd, Campbell MS, Iyer S, Roubin GS, Vitek J, Mathur A, et al. Extracranial vertebral artery stent placement: in-hospital and follow-up results. J Neurosurg. 1999. 91:547–552.
19. Taylor RA, Siddiq F, Suri MF, Martin CO, Hayakawa M, Chaloupka JC. Risk factors for in-stent restenosis after vertebral ostium stenting. J Endovasc Ther. 2008. 15:203–212.
20. Horlitz M, Amin FR, Boerrigter G, Sigwart U, Clague JR. Restenosis after successful ostial stent implantation: the role of statins compared with conventional treatment. J Interv Cardiol. 2004. 17:301–306.
21. Park DW, Hong MK, Suh IW, Hwang ES, Lee SW, Jeong YH, et al. Results and predictors of angiographic restenosis and long-term adverse cardiac events after drug-eluting stent implantation for aorto-ostial coronary artery disease. Am J Cardiol. 2007. 99:760–765.
22. Phipp LH, Scott DJ, Kessel D, Robertson I. Subclavian stents and stent-grafts: cause for concern? J Endovasc Surg. 1999. 6:223–226.
23. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999. 282:2035–2042.
24. Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001. 142:314–323.
25. Lin YH, Hung CS, Tseng WY, Lee RK, Wang YC, Lin MS, et al. Safety and feasibility of drug-eluting stent implantation at vertebral artery origin: the first case series in Asians. J Formos Med Assoc. 2008. 107:253–258.
26. Drescher R, Mathias KD, Jaeger HJ, Bockisch G, Demirel E, Gissler HM, et al. Clinical results of carotid artery stenting with a nitinol self-expanding stent (SMART stent). Eur Radiol. 2002. 12:2451–2456.
27. Linfante I, Hirsch JA, Selim M, Schlaug G, Caplan LR, Reddy AS. Safety of latest-generation self-expanding stents in patients with NASCET-ineligible severe symptomatic extracranial internal carotid artery stenosis. Arch Neurol. 2004. 61:39–43.
28. Wakhloo AK, Tio FO, Lieber BB, Schellhammer F, Graf M, Hopkins LN. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol. 1995. 16:1043–1051.
29. Vos AW, Linsen MA, Diks J, Rauwerda JA, Wisselink W. Carotid stent mobility with regard to head movements: in vitro analysis. Vascular. 2004. 12:369–373.
30. Kern MJ, Ouellette D, Frianeza T. A new technique to anchor stents for exact placement in ostial stenoses: the stent tail wire or Szabo technique. Catheter Cardiovasc Interv. 2006. 68:901–906.
31. Cheema A, Hong T. Buddy wire technique for stent placement at non-aorto ostial coronary lesions. Int J Cardiol. 2007. 118:e75–e80.
32. Divani AA, Berezina TL, Zhou J, Pakdaman R, Suri MF, Qureshi AI. Microscopic and macroscopic evaluation of emboli captured during angioplasty and stent procedures in extracranial vertebral and internal carotid arteries. J Endovasc Ther. 2008. 15:263–269.
33. Kantarci F, Mihmanli I, Albayram MS, Barutca H, Gulsen F, Kocer N, et al. Follow-up of extracranial vertebral artery stents with Doppler sonography. AJR Am J Roentgenol. 2006. 187:779–787.
34. Yoo WJ, Lim YS, Ahn KJ, Choi BG, Kim JY, Kim SH. Assessment of vertebral artery stents using 16-slice multi-detector row CT angiography in vivo evaluation: comparison of a medium-smooth kernel and a sharp kernel. Eur J Radiol. 2009. 70:362–368.
35. de Bray JM, Pasco A, Tranquart F, Papon X, Alecu C, Giraudeau B, et al. Accuracy of color-Doppler in the quantification of proximal vertebral artery stenoses. Cerebrovasc Dis. 2001. 11:335–340.
36. Suh DC, Kim SJ, Lee DH, Kim W, Choi CG, Lee JH, et al. Outcome of endovascular treatment in symptomatic intracranial vascular stenosis. Korean J Radiol. 2005. 6:1–7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download