Abstract
We report a case of an adenoma arising from an ectopic adrenal gland mimicking a hepatocellular carcinoma in a heavy alcohol abuser. A MDCT showed a 2.7 low-attenuating nodule in segment VII of the liver through all CT phases. Compared to a precontrast image, however, a subtle enhancement was noted on the arterial phase CT image. On T1 weighted in- and opposed-phase MR images, an abundant fat component within the lesion was seen. Dynamic contrast-enhanced MR images after administration of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) more clearly depicted hypervascularity and wash-out of the lesion on arterial and portal phases, respectively. On delayed hepatobiliary phase MR images, obtained 20 minutes after Gd-EOB-DTPA administration, subtle uptake or retention of the contrast agent by the lesion was suspected. A tumorectomy was performed and adrenal adenoma from an ectopic adrenal gland within the liver was confirmed.
Developmental abnormalities of the adrenal gland include ectopic or heterotopic adrenal tissues and adrenohepatic fusion. Ectopic adrenal tissues, also known as adrenal rest tissues, are usually found in close proximity to the adrenal glands, and along the path of descent or in association with the gonads because of the close spatial relationship between the adrenal and urogenital primordial (1). Ectopic adrenal tissues have also been reported in the gallbladder and liver (2, 3). Adrenohepatic fusion is defined as a fusion of the liver and right adrenal gland with closely intermingling parenchymal cells of these two organs (4). Its mechanism is known as the mesenchymal tissue defect which may lead to retardation of capsule formation between the two organs (5). Therefore, the presence of Glisson's capsule between the two organs is critical for the differentiation of the ectopic adrenal gland in the liver from adrenohepatic fusion.
There have been several reports on adrenal adenoma in adrenohepatic fusion tissue (6, 7). A very low density (≤ 10 HU) of the lesion on precontrast CT image and direct visualization of the fusion between the two organs on multiplanar reformatted images are helpful imaging features for the correct diagnosis. However, to the best of our knowledge, there are no reports on imaging findings of ectopic adrenal adenoma in the liver. Furthermore, gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA; Primovist, Bayer Schering Pharma, Berlin, Germany) enhanced MRI findings of adrenal adenoma has never been reported. Therefore, we report a case of a man suffering from alcoholism with adrenal adenoma arising from ectopic adrenal tissue in the liver mimicking hepatocellular carcinoma (HCC) on both MDCT and Gd-EOB-DTPA enhanced MRI.
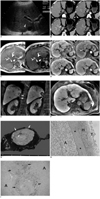
A 45-year-old man suffering from heavy alcoholism underwent an abdominal ultrasonography (US) during a routine check-up. On a transverse abdominal US image, echogenicity of the liver was diffusely increased, suggesting fatty infiltration in the liver. In addition, an approximately 2.7 cm low-echoic lesion was found in segment VII of the liver (Fig. 1A). For further evaluation, a dynamic contrast-enhanced liver CT was recommended. On a precontrast scan, a 2.5 cm well-demarcated, low-attenuating nodule was seen in the subcapsular area of hepatic segment VII (Fig. 1B). After contrast administration, the lesion showed heterogeneous enhancement on arterial and portal phase images and washout on delayed phase images (mean HU: -5 on precontrast phase, 27 on arterial, 89 on portal, and 46 on delayed phase) (Fig. 1B). The serologic tests were negative for the hepatitis B antigen and hepatitis C antibody. The level of alpha-fetoprotein was also normal (1.8 ng/mL). The tentative diagnosis at the time of interpretation included HCC with fatty metamorphosis, hepatic adenoma, and angiomyolipoma. A liver MRI with Gd-EOB-DTPA was performed for further characterization of the lesion. On in- and opposed-phase T1-weighted gradient recalled echo images, a marked signal drop of the lesion, as well as of the liver was noted, suggestive of an abundant fatty component within the lesion and the background liver (Fig. 1C). After Gd-EOB-DTPA administration, the lesion showed modest arterial enhancement on the arterial phase, and a washout pattern on the portal and equilibrium (3 minutes) phase images (Fig. 1D). Hepatobiliary phase images obtained 20 minutes after contrast injection demonstrated a clear defect of the lesion compared to the hyperintense background liver. However, a subtle hyperintense focus was seen off-center of the lesion. A coronal multi-planar reformatted image obtained 15 minutes after contrast injection clearly depicts a relationship between the lesion and the right adrenal gland (Fig. 1E). Even though the hyperintense focus seen on hepatobiliary phase may occur due to the nonspecific retention of gadolinium, it also may indicate the possibility of hepatocyte-specific contrast uptake by the tumor (Fig. 1F). However, according to American Association for the Study of Liver Diseases (AASLD) guidelines (8), a biopsy should be the next step for diagnosis due to the patient's non-cirrhotic liver, normal level of alpha-fetoprotein, and atypical vascular pattern on CT. Therefore, a biopsy was performed. However, the pathologic diagnosis was epithelial cell neoplasm with clear cytoplasm and was not conclusive with a differential list of HCC, adrenal adenoma, and renal cell carcinoma. In addition to the patient's history of chronic alcohol abuse, the enhancement pattern of the lesion on MR dynamic images, and the possibility of hepatocyte-specific contrast uptake by the tumor, cannot completely exclude the diagnosis of HCC with fatty metamorphosis. Therefore, the patient underwent surgery. A tumorectomy was planned preoperatively. During the operation, the lesion was found to be located within the liver and was completely covered with the hepatic capsule. The right adrenal gland was slightly attached to the hepatic capsule; however, dissection between the liver and adrenal gland was easily performed. The gross specimen showed a 2.9 cm yellowish nodule within the liver (Fig. 1G). A microscopic examination showed that the nodule was well encapsulated and completely surrounded by hepatocytes, hence confirming its intrahepatic location (Fig. 1H). The tumor was confirmed as an adrenal cortical adenoma consisting of clear cells with abundant cytoplasm (Fig. 1H). A retrospective microscopic examination demonstrated that dilated vessels and an inner organizing hematoma with vascular proliferation off-center of the lesion corresponded to the area showing focal contrast uptake at 20 minute delayed MR images (Fig. 1I). The final pathologic diagnosis was an adrenal cortical adenoma arising from ectopic adrenal tissue in the liver.
Adrenal cortical adenoma is the most common benign neoplasm in the adrenal gland and treatment is usually not necessary unless it is symptomatic. Approximately 80% of adrenal adenomas show < 10 HU on an unenhanced CT and can be easily diagnosed as a lipid-rich adenoma without other sophisticated examinations (9). Even though adrenal adenoma has little or no fatty component, also known as a lipid-poor adenoma, it can be differentiated from non-adenomatous lesions through the calculation of the percentage enhancement washout with high sensitivity and specificity (9). According to Caoili et al. (9), the use of the percentage enhancement washout threshold of 60% allowed them to correctly diagnose 19 of 22 lipid-poor adenomas with 98% sensitivity and 92% specificity. In our case, even though the patient underwent the liver protocol CT, which contains unenhanced, arterial phase, portal phase, and equilibrium (3 minutes) phase, we were able to obtain 60% enhancement washout of the lesion. Had we calculated the percentage enhancement washout using 15 minute delayed phase CT images which are usually recommended for the characterization of adrenal lesions, the percentage might have been much greater than 60%. Therefore, if the lesion in our patient had been located at the right adrenal gland, the correct diagnosis could have easily been achieved due to its lipid-rich character and its high enhancement washout of greater than 60%. Consequently, unnecessary surgery could have been avoided. However, like in our case, adrenal adenomas arising from a heterotopic or ectopic gland can result in diagnostic dilemmas.
It is important to diagnose ectopic adrenal tissue or its neoplasm as noninvasively as possible, as they are usually asymptomatic and no treatment is necessary. However, there are three circumstances in which clinical problems may occur: first, ectopic adrenal tissue may become symptomatic if it produces hormones such as aldosterone, cortisol, or androgens, which can lead to the presentation of Cushing's syndrome, aldosteronoma, Conn's syndrome or extreme virilization (10-12). However, in our case, there was no hormone-related symptoms. Second, various kinds of neoplasms such as neuroblastoma, myelolipoma, adenoma, and oncocytoma may occur in ectopic adrenal tissue (10, 13, 14). Third, the ectopic adrenal tissue itself or abnormality in the ectopic tissue may mimic lymphadenopathy or tumors in other abdominal organs (6). Radiologists should be aware of this last potential occurrence in order to avoid unnecessary surgery. However, in our case, even if we could consider the unique location of the lesion (bare area of segment VII) and proximity of the lesion to the right adrenal gland at the time of interpretation, complete exclusion of the possibility of hepatic tumor, in particular, hepatic adenoma or carcinoma, would be difficult due to the patient's history of chronic alcohol abuse and a similar dynamic enhancement pattern of the lesion to hepatocellular adenoma or carcinoma. Furthermore, hyperintense focus within the lesion on 20-minute delayed MR hepatobiliary phase images led to greater confusion. When we retrospectively analyzed the microscopic slides of the lesion with our abdominal pathologist, there were dilated vessels and inner organizing hematoma with vascular proliferation off-center of the lesion, which corresponded to the area showing focal hyperintensity at 20-minutes delayed MR images. Considering the microscopic findings, the hyperintense area seen on the 20-minute delayed hepatobiliary phase images might be due to prolonged retention of the gadolinium component by dilated tumoral vasculature, and not due to the true uptake of EOB-DTPA by hepatocytes.
Even though there were several reports on adrenal adenoma in adrenohepatic fusion tissue (6, 7), our case is the first radiologic demonstration of adenoma arising from an ectopic adrenal gland in the liver. The tumor closely abutted the right adrenal gland on both the radiologic examination and the operative field. However, the microscopic finding revealed that the tumor was well encapsulated and completely surrounded by hepatic parenchyma, confirming its pure intrahepatic and ectopic location.
Despite of the difficulties in reaching the correct preoperative diagnosis of adenoma originating from an ectopic or heterotopic adrenal gland, close proximity of the lesion to the adrenal gland might provide radiologists with a clue for this potential occurrence, thereby possibly averting aggressive surgery such as a right posterior sectionectomy.
Figures and Tables
Fig. 1
Adrenal cortical adenoma arising from ectopic adrenal gland in liver.
A. Transverse US image shows 2.5 cm subtle hypoechoic nodule (arrows) in segment VII of liver.
B. On unenhanced CT image (left upper), lesion (arrow) shows marked low attenuation and its CT attenuation number was -4 HU, suggesting presence of fat within lesion. After contrast administration, lesion (arrow) showed heterogeneous enhancement on arterial (right upper) and portal (left lower) phase images, as well as clear washout on delayed (right lower) phase images. CT attenuation numbers were 29, 82, and 30 on three dynamic phases, respectively.
C. On in- (left) and opposed (right) phase T1-weighted gradient recalled echo MR images, marked signal drop of lesion (arrow) as well as in liver is demonstrated, which suggests abundant fatty component within lesion and background liver, respectively.
D. After Gd-EOB-DTPA administration, lesion (arrow) shows modest enhancement on arterial phase (right upper) compared to unenhanced image (left upper) and washout pattern on portal (left lower) and 3-minute equilibrium (right lower) phase images.
E. Coronal MR images obtained 15 minutes after contrast injection clearly depict relationship between lesion (*) and right adrenal gland (arrowheads). Thin hyperintense capsule is clearly seen between two (in right image).
F. Hepatobiliary phase image obtained 20 minutes after contrast injection demonstrates clear defect of lesion (arrow) compared to hyperintense background liver. However, subtle hyperintense focus (arrowhead) is seen off-center of lesion, indicating possibility of contrast uptake by tumor. Also note thin hyperintense rim (thin arrows) by hepatocytes at medial aspect of lesion, suggesting intrahepatic location of lesion.
G. Gross pathologic specimen reveals 2.9 × 2.8 × 2.5 cm yellowish nodule surrounded by normal liver parenchyma and liver capsule (arrows). Note small foci of hemorrhage (arrowhead) off-center of lesion corresponding to area showing focal hyperintensity on 20-minute delayed MR images.
H. Microscopic photograph (original magnification ×200, Hematoxylin & Eosin staining) shows well encapsulated nodule (A) surrounded by hepatocytes (H) and separated from adjacent perihepatic tissue (T) by fibrotic capsule (*) and hepatocytes.
I. On low-power field microscopic photograph (original magnification ×40, Hematoxylin & Eosin staining), adrenal adenoma exhibits clear, lipid-laden cells arranged in sheets or nests (A) and contains organizing hematoma (arrows) in dilated vessel with inner vascular proliferation (*) corresponding to focal contrast uptake area on 20-minute delayed MR images.
References
1. Lack EE, Kozakewich HPW. Lack EE, editor. Embryonal, developmental anatomy, and selected aspects of non-neoplastic pathology. Pathology of the adrenal glands. 1990. New York, Edinburgh, London, Melbourne: Churchill Livingstone;1–74.
2. Busuttil A. Ectopic adrenal within the gall-bladder wall. J Pathol. 1974. 113:231–233.
3. Vestfrid MA. Ectopic adrenal cortex in neonatal liver. Histopathology. 1980. 4:669–672.
4. Honma K. Adreno-hepatic fusion. An autopsy study. Zentralbl Pathol. 1991. 137:117–122.
5. Honoré LH, O'Hara KE. Combined adrenorenal fusion and adrenohepatic adhesion: a case report with review of the literature and discussion of pathogenesis. J Urol. 1976. 115:323–325.
6. Woo HS, Lee KH, Park SY, Han HS, Yoon CJ, Kim YH. Adrenal cortical adenoma in adrenohepatic fusion tissue: a mimic of malignant hepatic tumor at CT. AJR Am J Roentgenol. 2007. 188:W246–W248.
7. Park BK, Kim CK, Jung BC, Suh YL. Cortical adenoma in adrenohepatic fusion tissue: clue to making a correct diagnosis at preoperative computed tomography examination. Eur Urol. 2009. [Epub ahead of print].
8. Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.
9. Caoili EM, Korobkin M, Francis IR, Cohan RH, Platt JF, Dunnick NR, et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002. 222:629–633.
10. Leibowitz J, Pertsemlidis D, Gabrilove JL. Recurrent Cushing's syndrome due to recurrent adrenocortical tumor--fragmentation or tumor in ectopic adrenal tissue? J Clin Endocrinol Metab. 1998. 83:3786–3789.
11. Medeiros LJ, Anasti J, Gardner KL, Pass HI, Nieman LK. Virilizing adrenal cortical neoplasm arising ectopically in the thorax. J Clin Endocrinol Metab. 1992. 75:1522–1525.
12. Gutowski WT 3rd, Gray G Jr. Ectopic adrenal in inguinal hernia sacs. J Urol. 1979. 121:353–354.
13. Stein SH, Latour F, Frost SS. Myelolipoma arising from ectopic adrenal cortex: case report and review of the literature. Am J Gastroenterol. 1986. 81:999–1001.
14. Maschler I, Rosenmann E, Ehrenfeld EN. Ectopic functioning adrenocortico-myelolipoma in longstanding Nelson's syndrome. Clin Endocrinol (Oxf). 1979. 10:493–497.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download