Abstract
Objective
The purpose of this study was to evaluate the technical feasibility and clinical efficacy of percutaneous transabdominal treatment of endoleaks after endovascular aneurysm repair.
Materials and Methods
Between 2000 and 2007, six patients with type I (n = 4) or II (n = 2) endoleaks were treated by the percutaneous transabdominal approach using embolization with N-butyl cyanoacrylate with or without coils. Five patients underwent a single session and one patient had two sessions of embolization. The median time between aneurysm repair and endoleak treatment was 25.5 months (range: 0-84 months). Follow-up CT images were evaluated for changes in the size and shape of the aneurysm sac and presence or resolution of endoleaks. The median follow-up after endoleak treatment was 16.4 months (range: 0-37 months)
Results
Technical success was achieved in all six patients. Clinical success was achieved in four patients with complete resolution of the endoleak confirmed by follow-up CT. Clinical failure was observed in two patients. One eventually underwent surgical conversion, and the other was lost to follow-up. There were no procedure-related complications.
Endoleaks represent one of the most common major complications encountered after endovascular aneurysm repair. Management of endoleaks remains somewhat controversial. Secondary intervention is mandatory in most cases with a type I endoleak because of the high risk of rupture (1, 2). Usually, type II endoleaks with a growing aneurysm sac are treated, while those with a shrinking sac are observed (3). However, the most effective methods for managing type II endoleaks are a matter of debate.
Transcatheter embolization of endoleaks is a less invasive treatment technique; it may provide a better approach to patient management than open surgical repair. Embolization can be performed through the transarterial approach or direct percutaneous puncture of the aneurysm sac via the translumbar (left side) or transcaval (right side) approaches. However, few cases of transabdominal embolization have been reported for repair of endoleaks (4-6). In this study, we report our experience with embolization using a percutaneous transabdominal approach for the treatment of type I and II endoleaks.
Between 2000 and 2007, 141 patients underwent endovascular aneurysm repair at our institution, and 33 endoleaks (23%) occurred. Based on follow-up imaging, the endoleaks were categorized as type I in 12 patients (9%), type II in 16 patients (11%), type III in two patients (1%), type IV in one patient (1%), and type V in two patients (1%). Initial treatment methods for the endoleaks are described in Table 1. Among the 33 patients with endoleaks, six patients (five men, one woman; age range, 61-81 years; mean age, 68.2 years) with type I or type II endoleaks underwent embolization by the transabdominal approach using a liquid embolic agent, N-butyl cyanoacrylate. Five patients had an aortoiliac bifurcated stent-graft due to an infrarenal abdominal aortic aneurysm, and one patient (No. 3) had an aortoiliac bifurcated stent-graft due to a ruptured abdominal aortic aneurysm. Four patients had a type I endoleak, and two had a type II endoleak. Five patients underwent a single session and one patient (No. 1) underwent two sessions of embolization.
The indications for treatment of a type I or II endoleak, at our institution, were evidence of a type I endoleak during follow-up; or a type II endoleak with a significant increase in the diameter of the aneurysm sac (≥ 5 mm difference by CT in the largest minor axis cross-sectional diameter of the aneurysm sac). Indications for a transabdominal procedure included a type I endoleak with failed alternative endovascular options (n = 4; No. 1-4) and a type II endoleak located at the anterior aspect of the stent-graft with an enlarging endoleak sac (n = 2; No. 5, 6). The failed endovascular procedures included balloon percutaneous angioplasty (n = 4; No. 1-4); aortic extender cuff (stent-graft) (Zenith, Cook, Bloomington, IN) (n = 2; No. 3, 4), and Palmaz stent placement (Johnson & Johnson Interventional System, Warren, NY) (n = 1; No. 3). Combined coil embolization was necessary in two patients to achieve repair of the endoleaks in cases of high-flow massive endoleaks from the attachment site (No. 3, 4) and in one case where the inferior mesenteric artery acted as an exit route for the endoleak (No. 3). Before the procedure, all patients received a medical evaluation and were determined to be good candidates for the transabdominal procedure. The median time between the endovascular aneurysm repair and embolization was 25.5 months (range: 0-84 months). The median follow-up after embolization was 16.4 months (range: 0-37 months).
Before the endovascular aneurysm repair, patients underwent CT imaging including pre-contrast, arterial phase, and 30-second delayed contrast enhanced images. Patients had follow-up CT with the same protocol at 30 days; 3, 6, and 12 months; and yearly thereafter. The largest minor axis cross-sectional diameter of the aneurysm sac was measured. A size greater than or equal to a 5-mm difference, by the CT imaging, in the largest minor axis cross-sectional diameter of the aneurysm sac was considered clinically significant.
Before percutaneous transabdominal embolization, preoperative aortography and selective angiography were performed via the transarterial approach. If treatment of the endoleak via the transarterial approach failed, a percutaneous transabdominal approach was immediately attempted. Before the procedure, intravenous prophylactic antibiotics (Cefazolin® 1 g [Yuhan Corp., Seoul, Korea] and Tobramycin 100 mg [Daewoong Pharmaceutical Co., Seoul, Korea]) were given. With the patient in the supine position, local anesthesia was administered at the puncture site of the abdomen. The target site was identified as a contrast-enhancing area of the aneurysm sac by CT (skin puncture point, puncture angle, depth of aneurysm sac from the skin) and color-flow ultrasound (US) guidance was used. The endoleak sac was punctured using a 21-gauge puncture needle (Chiba; Cook, Bloomington, IN) under fluoroscopic and/or color-flow US guidance. Bony landmarks and stent-graft marking bars were also referenced under fluoroscopic guidance. In case of bowel interposition at the anterior aspect of the aneurysm sac, the sigmoid and transverse colon were filled with barium, and the transabdominal approach was performed under fluoroscopic guidance to avoid colon injury. After confirmation of arterial blood flowing through the puncture needle, contrast media was injected to visualize the endoleak sac, a 0.018-inch guidewire was inserted, and the puncture tract was dilated. Over the 0.035-inch guide wire, a 5-Fr angiographic catheter was placed within the endoleak site, and an angiogram of the aneurysm sac was performed to evaluate the origin and outflow of the endoleak.
Embolization of the endoleak sac and outflow vessels was performed. A 2.8-Fr microcatheter (Progreat; Terumo, Tokyo, Japan) was used when a more advanced endoleak selection was necessary or when the selection of outflow vessels was attempted. Before injection of N-butyl cyanoacrylate (Histoacryl, B. Braun, Tuttlingen, Germany), the catheter was flushed with 5% dextrose-water solution to prevent precipitation. N-butyl cyanoacrylate liquid adhesive was mixed with iodized oil (Lipiodol Ultra Fluid, Guerbet, Aulnay-sous-Bois, France), ranging from 25% to 50% depending on the amount and velocity of blood flow from the endoleak. After embolization, the puncture tract was embolized using the N-butyl cyanoacrylate mixture.
The endpoint of the procedure was the nonvisualization of blood flow within the aneurysm sac on a completion transarterial angiogram. Technical success was defined as successful embolization of the endoleak sac and complete resolution of the endoleak on completion angiography. Clinical success was defined as complete resolution of the endoleak without enlargement of the aneurysm sac on follow-up CT.
Technical success was achieved in all six patients. One patient (No. 1) required a second embolization session. Clinical success was achieved in four patients. One patient (No. 3) had decreased diameter of the aneurysm sac, while three patients (No. 1, 5, 6) had an unchanged aneurysm diameter. Two cases of clinical failure occurred (No. 2, 4); they had persistent type I endoleaks with increased diameter of the aneurysm sac on follow-up CT for which surgical conversion was recommended. One patient (No. 2) eventually underwent surgical conversion, and the other patient (No. 4) was lost to follow-up. Combined coil embolization was required for endoleak sac embolization (No. 5) and for both sac and inferior mesenteric artery embolization (No. 4). The results of the transabdominal embolization for the treatment of endoleaks are summarized in Figure 1.
There were no procedure-related complications such as intraperitoneal bleeding, ischemic bowel injury, bowel perforation, or infection in the aneurysm sac or graft. Patient No. 3, an 81-year-old man who had a successful embolization, died eight months after the procedure due to sudden cardiac death that was unrelated to the procedure (Fig. 2, 3).
Compared with open repair of infrarenal abdominal aortic aneurysms, endovascular aneurysm repair is less invasive and results in significantly better perioperative outcomes, including fewer systemic complications, shorter operative time, lower use of postoperative mechanical ventilation, and shorter hospital stay, resulting in a lower risk of perioperative mortality (7, 8). However, secondary interventions are common in patients after endovascular aneurysm repair, and new complications such as incomplete exclusion of blood flow to the aneurysm sac, defined as an endoleak by White et al. (9), have led to the stent-graft procedure. Even though the technical innovation for of the stent-graft has been performed (10), correction of endoleaks is one of the major causes of secondary intervention during the primary admission or within 30 days of aneurysm repair (7).
Clinical management of endoleaks varies according to the different types. Type I and III endoleaks require treatment without delay, but the efficacy of management for type II endoleaks is a subject of debate (3, 11-14). At our institution, type II endoleaks are treated only when the aneurysm sac has grown by 5 mm or more during follow-up. Conventional methods for the management of type I endoleaks are stent-graft extensions, cuffs, or Palmaz stents. Stent-graft extensions or cuffs can be applied only if sufficient native aorta is available proximally or distally to support the stent (15-17). When conventional methods fail or devices are unavailable, N-butyl cyanoacrylate embolization via a transabdominal approach can be attempted before resorting to open surgical repair. The conventional approach for a type II endoleak is transarterial (18-20). If transarterial approaches fail, translumbar or transcaval approaches are usually used (21-28). However, if the endoleak sac is located at the anterior aspect of the stent-graft, translumbar or transcaval approaches are less feasible, and a transabdominal approach may be warranted.
All four patients with a type I endoleak had secondary endovascular interventions to repair the type I endoleaks. Two patients (No. 3, 4) had a sufficient margin for stent-graft extension. In patient No. 3, both an aortic extender cuff and a Palmaz stent were placed. In patient No. 4, an aortic extender cuff was placed. Two patients (No. 1, 2) had no margin for placement of an aortic extender cuff for the proximal type I endoleak, and no Palmaz stent was available; therefore, balloon percutaneous angioplasty was performed at the attachment site. These secondary endovascular interventions had failed to repair the type I endoleaks in all four patients with type I endoleaks. Transarterial embolization was attempted in two patients (No. 1, 2) as the next step. Patient No. 2 underwent transarterial embolization using N-butyl cyanoacrylate. However, the type I endoleak recurred on the 3-month follow-up CT. The transarterial embolization of the endoleak sac failed in patient No. 1.
In two patients with a type II endoleak, transarterial embolization failed in patient No. 5 because of tortuous tracks from the internal iliac artery. In patient No. 6, a new type II endoleak developed despite previously successful transarterial embolization for the type II endoleak, on the 6-month follow-up CT. Open surgical repair or percutaneous transabdominal embolization was recommended for all six patients. Because their general condition was unfavorable for major surgery and they refused surgical repair, embolization of the aneurysm sac was performed.
The advantage of direct endoleak embolization by the translumbar or transcaval approach is that these methods avoid traversing cavities or organs (21-29). Therefore, these approaches are ideal when an endoleak is located at the posterior aspect of the endovascular stent-graft or when bowel or another organ is interposed (27). However, when the endoleak sac is located at the anterior aspect of the endovascular stent-graft, an anterior transabdominal approach is needed for embolization of the endoleak sac. The transabdominal approach has the advantage of being performed with the patient in the supine position, so that transarterial angiography can be performed simultaneously. Moreover, immediate confirmation of the angiography results and accurate targeting can be achieved. The risk of organ injury during the transabdominal approach can be minimized when performed under fluoroscopic and/or real-time US guidance.
There were two cases with clinical failure (No. 2, 4). Patient No. 2, who originally had a type I endoleak, underwent an aortic extender cuff placement for repair of a type I endoleak; however, a persistent type I endoleak was observed during follow-up on CT. For treatment of this endoleak, transarterial embolization was performed with insufficient exclusion of the endoleak. The transabdominal approach was performed for the next procedure and there was no visible endoleak on final aortography. However, increased diameter of the aneurysm sac was observed on the 1-year follow-up CT, raising the possibility of a recurrent type I endoleak. This case met our definition of a clinical failure, and surgical repair was recommended; at surgery, there was no definite evidence of an endoleak. We assumed that the cause of the increased diameter of the aneurysm sac was a type V endoleak or a subtle type I endoleak. Patient No. 4, who also originally had a type I endoleak, underwent aortic extender cuff placement for the repair of the type I endoleak; however, a persistent type I endoleak was noted during follow-up CT. When percutaneous transabdominal embolization was performed, the type I endoleak was not completely repaired and a patulous type I endoleak resulted. A percutaneous approach was inappropriate in this case with a patulous type I endoleak. Surgical repair was recommended.
The results of this study suggest that when treatment of an endoleak is considered necessary, and when the endoleak sac is located at the anterior aspect of the stent-graft, a percutaneous transabdominal approach to embolization under both color-flow US and fluoroscopic guidance is warranted. The percutaneous transabdominal procedure for the treatment of type I or II endoleaks after endovascular aneurysm repair is technically feasible and should be considered an alternative method when conventional endovascular methods have failed.
Figures and Tables
Fig. 2
Steps for endoleak repair via transabdominal approach with type l endoleak, 59-year-old patient with type l endoleak (No. 2).
A. Preprocedural CT showed type l endoleak (black arrows) within aneurysm sac. Inferior mesenteric artery (white arrow) was exit route for endoleak. Area previously treated with N-butyl cyanoacrylate via transarterial approach for repair of type l endoleak can be seen (asterisk).
B. Digital subtraction angiography via transabdominal approach showed type l endoleak (black arrow) with inferior mesenteric artery (white arrows). Embolization of endoleak sac using N-butyl cyanoacrylate was performed (not shown).
C. One-month follow-up CT showed complete repair of type l endoleak with radiopaque N-butyl cyanoacrylate in place of previous endoleak sac. However, 1-year follow-up CT (not shown) showed increased diameter of aneurysm sac with indistinct type l endoleak and patient eventually underwent surgical conversion.
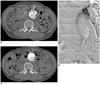
Fig. 3
Steps for endoleak repair via transabdominal approach with type ll endoleak, 64-year-old patient with type ll endoleak (No. 6).
A. Preprocedural CT showed location of type ll endoleak (arrow) within aneurysm sac.
B. Digital subtraction angiography delineated size and structure of type ll endoleak, accessed via retrograde catheterization of inferior mesenteric artery.
C. Embolization of endoleak sac using N-butyl cyanoacrylate was done by transarterial approach via inferior mesenteric artery.
D. Recurrence of new type ll endoleak (white arrow) that communicated with lumbar artery (black arrow) developed after six months of follow-up.
E. Digital subtraction angiography showed endoleak sac communicating with lumbar artery (arrows). Transabdominal approach was performed since main endoleak sac was located anteriorly.
F. Embolization of endoleak sac was performed using N-butyl cyanoacrylate.
G. 3-year follow-up CT demonstrated complete repair of type ll endoleak with radiopaque N-butyl cyanoacrylate in place of previous endoleak sac.
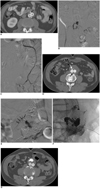
Table 1
Initial Treatment Methods for Endoleak in 33 Patients

Note.-Transarterial approach includes conventional approach of endoleak treatment such as stent-graft extensions, cuffs, and Palmaz stent in cases with type l endoleak, and transarterial embolization of dominant feeding artery such as inferior mesenteric artery or lumbar arteries in cases with type ll endoleak.
References
1. White GH, Yu W, May J, Chaufour X, Stephen MS. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997. 4:152–168.
2. Buth J, Harris PL, van Marrewijk C, Fransen G. The significance and management of different types of endoleaks. Semin Vasc Surg. 2003. 16:95–102.
3. Baum RA, Carpenter JP, Tuite CM, Velazquez OC, Soulen MC, Barker CF, et al. Diagnosis and treatment of inferior mesenteric arterial endoleaks after endovascular repair of abdominal aortic aneurysms. Radiology. 2000. 215:409–413.
4. Boks SS, Andhyiswara T, de Smet AA, Vroegindeweij D. Ultrasound-guided percutaneous transabdominal treatment of a type 2 endoleak. Cardiovasc Intervent Radiol. 2005. 28:526–529.
5. Kasthuri RS, Stivaros SM, Gavan D. Percutaneous ultrasound-guided thrombin injection for endoleaks: an alternative. Cardiovasc Intervent Radiol. 2005. 28:110–112.
6. Ellis PK, Kennedy PT, Collins AJ, Blair PH. The use of direct thrombin injection to treat a type II endoleak following endovascular repair of abdominal aortic aneurysm. Cardiovasc Intervent Radiol. 2003. 26:482–484.
7. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004. 364:843–884.
8. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004. 351:1607–1618.
9. White GH, Yu W, May J. Endoleak--a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg. 1996. 3:124–125.
10. Sun Z, Mwipatayi BP, Allen YB, Hartley DE, Lawrence-Brown MM. Multislice CT angiography of fenestrated endovascular stent grafting for treating abdominal aortic aneurysms: a pictorial review of the 2D/3D visualizations. Korean J Radiol. 2009. 10:285–293.
11. Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. European Collaborators on Stent/graft techniques for aortic aneurysm repair. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg. 2000. 32:739–749.
12. Baum RA, Stavropoulos SW, Fairman RM, Carpenter JP. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003. 14:1111–1117.
13. Steinmetz E, Rubin BG, Sanchez LA, Choi ET, Geraghty PJ, Baty J, et al. Type II endoleak after endovascular abdominal aortic aneurysm repair: a conservative approach with selective intervention is safe and cost-effective. J Vasc Surg. 2004. 39:306–313.
14. van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J. EUROSTAR Collaborators. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004. 27:128–137.
15. Maldonado TS, Rosen RJ, Rockman CB, Adelman MA, Bajakian D, Jacobowitz GR, et al. Initial successful management of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg. 2003. 38:664–670.
16. Golzarian J, Maes EB, Sun S. Endoleak: treatment options. Tech Vasc Interv Radiol. 2005. 8:41–49.
17. Faries PL, Cadot H, Agarwal G, Kent KC, Hollier LH, Marin ML. Management of endoleak after endovascular aneurysm repair: cuffs, coils, and conversion. J Vasc Surg. 2003. 37:1155–1161.
18. Kasirajan K, Matteson B, Marek JM, Langsfeld M. Technique and results of transfemoral superselective coil embolization of type II lumbar endoleak. J Vasc Surg. 2003. 38:61–66.
19. LaBerge JM, Sawhney R, Wall SD, Chuter TA, Canto CJ, Wilson MW, et al. Retrograde catheterization of the inferior mesenteric artery to treat endoleaks: anatomic and technical considerations. J Vasc Interv Radiol. 2000. 11:55–59.
20. van Schie G, Sieunarine K, Holt M, Lawrence-Brown M, Hartley D, Goodman MA, et al. Successful embolization of persistent endoleak from a patent inferior mesenteric artery. J Endovasc Surg. 1997. 4:312–315.
21. Binkert CA, Alencar H, Singh J, Baum RA. Translumbar type II endoleak repair using angiographic CT. J Vasc Interv Radiol. 2006. 17:1349–1353.
22. Baum RA, Cope C, Fairman RM, Carpenter JP. Translumbar embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2001. 12:111–116.
23. Stavropoulos SW, Kim H, Clark TW, Fairman RM, Velazquez O, Carpenter JP. Embolization of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms with use of cyanoacrylate with or without coils. J Vasc Interv Radiol. 2005. 16:857–861.
24. van den Berg JC, Nolthenius RP, Casparie JW, Moll FL. CT-guided thrombin injection into aneurysm sac in a patient with endoleak after endovascular abdominal aortic aneurysm repair. AJR Am J Roentgenol. 2000. 175:1649–1651.
25. Rial R, Serrano Fj F, Vega M, Rodriguez R, Martin A, Mendez J, et al. Treatment of type II endoleaks after endovascular repair of abdominal aortic aneurysms: translumbar puncture and injection of thrombin into the aneurysm sac. Eur J Vasc Endovasc Surg. 2004. 27:333–335.
26. Martin ML, Dolmatch BL, Fry PD, Machan LS. Treatment of type II endoleaks with Onyx. J Vasc Interv Radiol. 2001. 12:629–632.
27. Stavropoulos SW, Carpenter JP, Fairman RM, Golden MA, Baum RA. Inferior vena cava traversal for translumbar endoleak embolization after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2003. 14:1191–1194.
28. Mansueto G, Cenzi D, D'Onofrio M, Petrella E, Gumbs AA, Mucelli RP. Treatment of type II endoleaks after endovascular repair of abdominal aortic aneurysms: transcaval approach. Cardiovasc Intervent Radiol. 2005. 28:641–645.
29. Kirby L, Goodwin J. Treatment of a primary type IA endoleak with a liquid embolic system under conditions of aortic occlusion. J Vasc Surg. 2003. 37:456–460.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download