Abstract
A percutaneous transthoracic needle biopsy is a common procedure in the practice of pulmonology. An air embolism is a rare but potentially fatal complication of a percutaneous transthoracic needle biopsy. We report four cases of a cerebral air embolism that developed after a percutaneous transthoracic needle biopsy. Early diagnosis and the rapid application of hyperbaric oxygen therapy is the mainstay of therapy for an embolism. Prevention is the best course and it is essential that possible risk factors be avoided.
Apercutaneous transthoracic needle biopsy (PCNB) is a common interventional radiological procedure, but has several associated complications. The most frequent complications are pneumothorax (27%), intrapulmonary hemorrhage (11%) and hemoptysis (7%), which are usually mild and self-limiting (1, 2). However, it is known that less frequent complications such as severe hemorrhage and an air embolism are potentially fatal (1, 2). Here we report four cases of a cerebral air embolism after a PCNB that occurred from July 1998 to March 2007.
A 40-year-old seaman presented with malaise, fever, dyspnea and a 10 kg weight loss (15% of body weight over a period of the months). High-resolution computed tomography (CT) images demonstrated diffuse ground glass opacities (GGO) in both lungs. A transbronchial lung biopsy (TBLB) was performed but was complicated by the presence of pneumothorax (Fig. 1A). However, as the size of the biopsy sample was inadequate, PCNB was performed immediately after the TBLB. Under CT guidance with the patient in the supine position, a chest radiologist with 18 months of experience performed percutaneous aspiration with the use of a 22-gauge 15-cm Chiba biopsy needle (InterV; Angiotech, Gainesville, FL). A CT scan showed that the needle was placed into the right lung lesion. The procedure was successful at the first attempt. Just after aspiration, the patient complained of dyspnea and subsequently a tonic-clonic type seizure developed with anisocoric pupil dilation. Brain CT imaging was performed with application of an oxygen mask and showed abnormal air densities in the sulci of both the parietal and occipital lobes (Fig. 1B). Unfortunately, hyperbaric oxygen therapy (HBOT) was not available at that time. The PCNB specimen showed the presence of Pneumocystis carinii organisms, and serum testing was positive for antibodies against human immunodeficiency virus (HIV) and the CD4 cell count was less than 50 cells/mm3. Accordingly, the final diagnosis was acquired immunodeficiency syndrome (AIDS) with Pneumocystis carinii pneumonia. Transthoracic echocardiography (TTE) performed two days later failed to reveal any structural abnormalities that could cause the right-to-left shunt. Despite supportive management and antibiotics, the patient died of pneumonia and sepsis without any improvement in mental status.
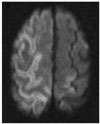
A 75-year-old man with chest pain was referred for the evaluation of a lung mass. Under CT guidance with the patient in the supine position, a chest radiologist with six months of experience performed a PCNB. An 18-gauge ACN biopsy needle (Angiotech) was used and the needle depth was verified by the use of a CT scan. The patient inspired during the procedure against instruction. Immediately after the procedure, the patient was unresponsive to stimulation and his four extremities became spastic. At that time the blood pressure was 80/60 mmHg, which recovered to 110/70 mmHg after fluid resuscitation. A neurological examination showed left-side hemiparesis and right-side spasticity. A brain CT scan demonstrated diffuse hypodensities in the right frontal and both parietal lobes. HBOT (100% oxygen, 2.2 atmospheres for 110 minutes) was initiated 170 minutes after the event. Brain MRI after three days showed findings consistent with an acute cerebral infarction in the right hemisphere (Fig. 2). A TTE was normal. The biopsy finding was a sarcomatoid carcinoma. Although neurological sequelae recovered slowly, the patient died of pneumonia with sepsis and respiratory failure at three months after the PCNB.
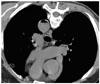
A 67-year-old man was referred for the evaluation of a lung lesion. The patient complained of a productive cough of three weeks duration and a chest CT scan showed the presence of multiple nodules with GGO in both lung fields. As fiber optic bronchoscopy with a TBLB was not diagnostic, a chest radiologist with two years of experience performed a CT-guided PCNB with the use of an 18-gauge ACN biopsy needle (Angiotech) in a patient placed in the supine position. However, the mental status of the patient deteriorated rapidly just after the biopsy, which was followed by convulsions and left side hemiparesis. A careful review of chest CT scans obtained during the procedure revealed the presence of air fluid level in the descending aorta (Fig. 3). Brain CT scans and MRI were checked, but no abnormality was evident. According to the temporal relationship between the neurological signs and the biopsy procedure, we made a presumptive diagnosis of a cerebral air embolism. Ninety hours after the event, the patient underwent HBOT (100% oxygen, 2.2 atmospheres for 60 minutes). The mental status improved after HBOT and one day later the patient underwent one additional HBOT session. A TTE was normal. The patient was discharged without any neurological sequelae.
A 67-year-old female was admitted for a PCNB of a growing lung nodule. A chest radiologist with 42 months of experience performed a PCNB under CT guidance. The biopsy needle used and the biopsy procedure were the same as described for case 2. Immediately after the procedure, the patient coughed up about 300 mL of blood, became hypotensive and lost consciousness. After fluid infusion and the application of an oxygen mask, the blood pressure and consciousness recovered but left-side hemiparesis had newly developed. A brain CT scan showed abnormal air densities in the cerebral sulci of the right fronto-parietal lobes. Within three hours of the event, the patient underwent HBOT (100% oxygen, 2.2 atmospheres for 110 minutes). Two days later the motor function improved and the patient was discharged after recovering fully. A TTE was normal. The biopsy finding was a chronic granulomatous inflammation.
A PCNB is commonly used to diagnose lung diseases. An air embolism is a rare but potentially fatal complication of a PCNB, and may cause subsequent myocardial infarction, intractable arrhythmia or stroke (2), and has an incidence rate of 0.02-0.07% after a PCNB (1). Recently, Hiraki et al. (3) reported four cases of a nonfatal systemic air embolism after a PCNB. These investigators routinely performed post-procedural scanning of the entire thorax to identify the potentially fatal complication and reported a rate of 0.4% (4 per 1,010 PCNBs). It was concluded that the incidence of a systemic air embolism is probably underestimated due to the missing of systemic air in asymptomatic patients. At our institute, about 250 PCNB's are performed annually, and we have diagnosed a cerebral air embolism in four cases over a ten-year period (roughly 0.16%). Although this level was not determined after a prospective study aimed at the determination of the precise incidence of this complication, the above level probably represents the incidence rate for clinically significant disease. The relatively high incidence rate of our institute may be attributable to our attention for air embolisms after first detection. To the best of our knowledge, few prospective studies that have shown the exact incidence of an air embolism have been performed as an air embolism is a rare complication.
There are three possible ways for air to be introduced into the pulmonary venous system during a PCNB. First, as air may enter directly through the needle if the needle tip is placed into a pulmonary vein while its base is open to the atmosphere and the atmospheric pressure exceeds the pulmonary venous pressure (as may occur during deep inspiration), then an air embolism occurs. One of the patients (case 2) was known to have inspired during the PCNB. Second, a needle may simultaneously penetrate an air-containing space and a nearby pulmonary vein, and coughing or the Valsalva maneuver may increase the airway pressure and facilitate the aspiration of air into the pulmonary vein (3, 4). Case 1 already had pneumothorax, and a PCNB should not have been performed on the same side. Case 4 had bronchiectatic changes near the lung lesion. In all cases, except for case 2, a PCNB was performed on the lung parenchyma and not on a solid tumor (Table 1). In addition, rigid lungs with cystic or cavitary lesions and vasculitis have also been described as risk factors for an air embolism (5, 6). Finally, air may enter into the pulmonary venous circulation from pulmonary arterial circulation by traversing the pulmonary microvasculature, even in the absence of an arteriovenous malformation (4). However, in the described cases, no anatomical abnormality was found by the use of TTE.
The most characteristic and diagnostic finding of cerebral air embolism is the visualization of gas bubbles in the cerebral arteries by the use of brain CT imaging. However, images often fail to reveal gas emboli, and therefore brain CT imaging cannot be routinely used after a PCNB. Accordingly, a high level of clinical suspicion is essential to ensure rapid diagnosis. Recently, Hirasawa et al. (7) reported that CT-fluoroscopy can visualize air entry during the procedure, and the investigators have suggested the use of CT fluoroscopy as a possible means of early detection.
Hyperbaric oxygen therapy is regarded as the mainstay of therapy for air embolisms. HBOT reduces bubble size and consequently removes occlusive bubbles and reduces endothelial damage (4). A literature review has reinforced the notion that the early application of HBOT is beneficial for patients with a cerebral air embolism (7, 8). However, delayed HBOT is probably also effective to increase the likelihood of survival and neurological recovery as the presence of air bubbles have been demonstrated up to 48 hours after the initial event (9). Furthermore, the different prognoses described in the present study are probably attributable to not only the amounts and locations of air emboli but also to the severity of the underlying lung disease.
It seems that an air embolism can occur regardless of needle size, the sampling method and the positioning of the patient (3). Occurrence of an air embolism is associated with several risk factors such as coughing, positive pressure ventilation during the procedure, a biopsy for a cystic or cavitary lesion and placement of the needle tip within the pulmonary vein. Therefore, the following steps can be a way to prevent this complication. First, the patient should be educated not to breathe during the procedure. Second, a PCNB should be avoided in risky patients who are unable to cooperate due to age or the presence of an intractable cough. Third, the length of time when the introducer needle is open to the atmosphere should be as short as possible, and the needle opening should be controlled with the use of a finger or a stylet. Finally, more attention should be given when a PCNB is performed for a cystic or cavitary lesion, lung parenchyma or GGO, since these lesions increase the risk of an air embolism. The lung parenchyma and GGO may include vessels and bronchi, and a cutting biopsy of these lesions often causes parenchymal hemorrhage that may induce coughing accompanied by an increasing risk of an air embolism (3, 5). However, as in the case reported by Arnold and Zwiebel (10), an air embolism can occur even when the technique is excellent and patient cooperation is adequate.
Although the incidence rate of a cerebral air embolism is low, its potential mortality requires that physicians be aware of this serious complication when performing a PCNB, and when clinically suspected, every effort should be made to achieve a rapid diagnosis and institute HBOT.
Figures and Tables
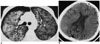 | Fig. 1High-resolution CT scan demonstrates diffuse ground glass attenuations in both lungs, and small amount of right pneumothorax due to previous transbronchial lung biopsy (A). Brain CT image shows abnormal air densities in sulci of parietal and occipital lobes (B). |
References
1. Sinner WN. Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn (Stockh). 1976. 17:813–828.
2. Laurent F, Montaudon M, Latrabe V, Begueret H. Percutaneous biopsy in lung cancer. Eur J Radiol. 2003. 45:60–68.
3. Hiraki T, Fujiwara H, Sakurai J, Iguchi T, Gobara H, Tajiri N, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy. Chest. 2007. 132:684–690.
4. Muth CM, Shank ES. Gas embolism. N Engl J Med. 2000. 342:476–482.
5. Aberle DR, Gamsu G, Golden JA. Fatal systemic arterial air embolism following lung needle aspiration. Radiology. 1987. 165:351–353.
6. Kodama F, Ogawa T, Hashimoto M, Tanabe Y, Suto Y, Kato T. Fatal air embolism as a complication of CT-guided needle biopsy of the lung. J Comput Assist Tomogr. 1999. 23:949–951.
7. Hirasawa S, Hirasawa H, Taketomi-Takahashi A, Morita H, Tsushima Y, Amanuma M, et al. Air embolism detected during computed tomography fluoroscopically guided transthoracic needle biopsy. Cardiovasc Intervent Radiol. 2008. 31:219–221.
8. Ohashi S, Endoh H, Honda T, Komura N, Satoh K. Cerebral air embolism complicating percutaneous thin-needle biopsy of the lung: complete neurological recovery after hyperbaric oxygen therapy. J Anesth. 2001. 15:233–236.
9. Wherrett CG, Mehran RJ, Beaulieu MA. Cerebral arterial gas embolism following diagnostic bronchoscopy: delayed treatment with hyperbaric oxygen. Can J Anaesth. 2002. 49:96–99.
10. Arnold BW, Zwiebel WJ. Percutaneous transthoracic needle biopsy complicated by air embolism. AJR Am J Roentgenol. 2002. 178:1400–1402.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download