Abstract
Choledochal cysts are rare congenital anomalies which are principally diagnosed by disproportional dilatation of the extrahepatic bile ducts. In addition, choledochal cysts are believed to arise from the anomalous union of the common bile duct and pancreatic duct outside the duodenal wall which is also proximal to the sphincter of the Oddi mechanism. The various types of choledochal cysts have been classified on the basis of these anomalous unions (Komi classification) and their anatomical locations (Todani classification). The multidetector computed tomography with reformatted imaging, magnetic resonance cholangiopancreatography, and an endoscopic retrograde cholangiography represent the important techniques providing the anatomical resolution and detail required to properly diagnose and classify choledochal cysts and their associated abnormal features of the biliary tree, as well as their pancreaticobile duct union. This study describes the various imaging features of a choledochal cyst in adults according to the various types of anomalous unions of the pancreaticobile duct according to Komi's classification and anatomic location according to Todani's classification. Lastly, we also review and discuss the associated abnormal findings developed in biliary systems.
Choledochal cysts are congenital cystic dilatations of any portion of the bile ducts, but most often occur in the main portion of the common bile duct (CBD). The diagnosis of a choledochal cyst is made on the basis of disproportional dilatation of the extrahepatic bile ducts (EHDs) after excluding the possibility of a tumor, stone, or inflammation as the cause of the dilatation.
The estimated incidence of choledochal cysts in Western countries varies between 1 in 100,000 and 1 in 150,000 individuals. The rate of incidence is higher in Asia and occurs more frequently in women (1 male: 4 female) (1). Though the diagnosis of a choledochal cyst is most often made during childhood, 25% of patients are initially seen as adults (2).
Abdominal pain, jaundice, and an abdominal mass are noted in most child cases. Although abdominal pain is the most common presenting symptom in adult cases (1, 3), most had nonspecific clinical symptoms (4). Further, secondary hepatobiliary disease in adults may obscure the primary condition.
An endoscopic retrograde cholangiopancreatography (ERCP) is generally regarded as the gold standard for the diagnosis of choledochal cysts and associated anomalies. However, the ERCP has inherent morbidity because of its invasiveness. Alternatively, the magnetic resonance cholangiopancreatography (MRCP) is a useful, noninvasive tool which shows good overall accuracy in the detection and classification of choledochal cysts (5). Furthermore, the MRCP can simultaneously and effortlessly delineate both the biliary and pancreatic duct (PD) structure, whereas the ERCP may fail to delineate these structures in patients with choledochal cysts due to the possibility of the tight structure in the distal portion of the cyst. Therefore, the MRCP may be a superior diagnostic tool over the ERCP for patients with choledochal cysts (5).
A multidetector computed tomography (MDCT) with reformatted imaging is another important technique which has the ability to demonstrate the anatomic details of the biliary tree and the pancreaticobile duct union (6). However, the MRCP and CT show a limited ability to detect certain minor ductal anomalies (5, 6). An endoscopic ultrasonography is less invasive than the ERCP and represents a more useful modality for identifying anomalies as well as some small and early lesions which are characteristics of combined complications (7).
Many theories exist about the etiology of choledochal cysts. In recent years, the focus has been drawn to the close association between the anomalous union of the pancreaticobile duct (AUPBD), and the formation of choledochal cysts (8). Developmentally, the CBD and PD normally unite within the sphincter of Oddi to form a common channel. The normal length of the common channel is 0.2-1.0 cm. It is postulated that the anomalous union of the CBD and the PD outside the duodenal wall and proximal to the sphincter of Oddi mechanism is responsible for choledochal cyst formation (7). The etiology is also believed to be biochemical in nature because the anomalous union produces an abnormal channel of less than 15 mm, which allows pancreatic enzymes to enter the biliary tree. This results in the weakening of the CBD wall and favors the formation of a cyst (9). Some other theories for partial duct obstruction include the mechanisms responsible for the sphincter of Oddi dysfunction, inadequate autonomic innervation, or the unequal vacuolization during organogenesis (10). All of these theories are interesting; however, support for these theories is sparse and, at best, only partially explain the pathogenesis since choledochal cysts can occur without an AUPBD. In addition, anomalous unions are common even in the absence of biliary disease. Additionally, pancreatic enzymes are not universally found within choledochal cysts (3). Therefore, it appears unlikely that a single mechanism is responsible for the various types of choledochal cysts and different mechanisms which may cause cysts in children and adults.
Two unique features of AUPBD are generally used to define choledochal cysts. The first feature is the formation of a long common channel, which results from the union of the PD and the CBD outside the duodenal wall. The second feature relates to the angle of the junction between the PD and the CBD. Normally, an acute angle forms between the PD and the distal CBD as they converge on, and are enclosed by the sphincter of Oddi.
Komi et al. (11) classified AUPBD into three types according to the angle of this ductal union (Fig. 1). Type I unions are defined by a right angle between the ducts along with sub-classifications type 1A and IB, based on the association without dilatation (1A) (Figs. 2, 3) or with dilatation (1B) (Fig. 4) of the common channel. Type II unions are defined by an acute angle between the ducts with sub-classifications type IIA and IIB based on the association without dilatation (IIA) (Fig. 5) or with dilatation (IIB) (Fig. 6A) of the common channel. Type III unions are defined by more complex patterns of accessory pancreatic ducts, with or without intricate duct networks. The sub-classifications include Type IIIA, which is equivalent to the classic pancreas divisum with biliary dilatation. Type IIIB is characterized by the absence of the Wirsung's duct. Type IIIC1 contains a tiny communicating duct between the main duct and the accessory ducts. Type IIIC2 (Fig. 7) is characterized by a common channel made up of common and accessory ducts of equal caliber. Finally, type IIIC3 (Fig. 8) is characterized by total or partial dilatation of the ductal system.
The widely accepted classification system for choledochal cysts, which was devised by Todani et al. (12) (Fig. 9), is based on the cholangiographic morphology, location, and number of intrahepatic and extrahepatic bile duct cysts. Type I cysts are confined to the extrahepatic bile duct (EHD) and can be further subdivided into IA (Fig. 3). Alternatively, a type I cyst involving the entire EHD can be further sub-classified into IB (Fig. 5), which involves only a focal segment of the EHD, and IC (Figs. 7, 10, 11) involving only the CBD. Type II cysts are true diverticula of the EHD. Type III cysts are also referred to as choledochocele and are confined to the EHD within the duodenal wall (Fig. 12). Type IV cysts have multiple features which can include both an extrahepatic and intrahepatic component. Type IV cysts can be further subdivided into type IVA involving both the EHD and intrahepatic bile duct (IHD) (Fig. 2), as well as type IVB, involving multiple segmental dilatations of the EHD. Finally, type V (Calori's disease) cysts are confined to the IHD (Fig. 13).
Type II lesions appear as a diverticulum of the CBD with some cases closely resembling gallbladder (GB) duplication. Furthermore, other cases resemble more rudimentary diverticular structures. Choledochoceles are a rare and easily overlooked anomaly with an unknown cause. Most observers consider choledochoceles as a variant of duodenal duplication. Its appearance resembles a protrusion of a dilated intramural segment of the CBD into the duodenum, which is analogous to the ureteroceles (Fig. 12A). Calori's disease is thought to be an inherited condition and is clinically associated with intrahepatic gallstone formation and a high incidence of renal disease. Although the radiological findings in Calori's disease may resemble those of a choledochal cyst, the other features show it to be a distinct, unrelated entity (3). Subsequently, Todani et al. (13) revised their classification of choleodochal cysts of type I based on pancreaticobiliary malunion. Type IA is a cystic dilatation of the EHD. Type IB is a segmental dilatation with no presence of pancreaticobiliary malunion. Type IC refers to a fusiform, diffuse, or cylindrical dilatation of the EHD with pancreaticobiliary malunion. Moreover, the dilatation often extends continuously into the intrahepatic duct. Lastly, type IVA shows primary ductal stricture, especially around the hepatic hilum (Fig. 7B) and umbilicus.
Visser et al. (3) recommended abandoning these numerical designations altogether so that classification of choledochal cysts could be simpler, easier to understand, and more specific to choledochal cysts rather than also encompassing some of the related anomalies. They recommended referring to the anomalies using the descriptive terms already in common use: choledochal cyst, choledochal diverticulum, choledochocele, and Caroli's disease with the term congenital choledochal cyst reserved for the condition made up of Todani types IA through IC and IVA. An added advantage to this system is that it would be unnecessary to include cases for the unrelated anomalies in discussions relating to congenital choledochal cysts.
Complications of choledochal cysts in adults include cholecystitis (Fig. 14), recurrent cholangitis, biliary stricture, choledocholithiasis (Figs. 4, 8), recurrent acute pancreatitis, and malignant transformation into cholangiocarcinoma (1). The incidence of biliary tract cancer in patients with choledochal cysts increases with age (4). Moreover, the diagnosis of adult choledochal cysts is frequently delayed due to nonspecific clinical symptoms or symptoms obscured by secondary hepatobiliary disease. Delays in diagnosis and treatment can often extend for years, which results in an increased risk of malignant degeneration. Malignant degeneration occurs most often in Todani type I (Figs. 3, 10) and IV cysts (1) (Fig. 6). While most of the reported cases of cancer arising in choledochal cysts are cholangiocarcinomas within dilated EHDs, 10% are GB cancer (14) (Figs. 5, 7, 10). GB cancer in patients with AUPBD and without CBD dilatation was more common than patients with GB cancer with choledochal cysts (15). Therefore, if a minor abnormality is detected in the wall of acalculous GB on ultrasonography, further testing should be performed to exclude AUPBD (15).
In the past, choledochal cysts were often treated using drainage procedures, however, the optimal treatment used today is likely to involve the complete excision of the extrahepatic duct, cholecystectomy, and Roux-en-Y hepaticojejunostomy. It has become clear that these operations have an unacceptable rate of long-term complications, most notably the malignant transformation (1, 2). However, a long term study is recommended for all patients with choledochal cysts.
Figures and Tables
 | Fig. 1Classification of anomalous union of pancreaticobile duct as per Komi et al. (11).
AP = accessory pancreatic duct, C = choledochal cyst, CC = common channel, D = duodenum, MP = main pancreatic duct, P = pancreatic duct
|
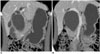 | Fig. 2Choledochal cyst with anomalous union of pancreaticobile duct (Komi IA, Todani IVA).
A, B. Coronal reformatted CT scans show cystic dilatation of common bile duct with segmental dilatation of proximal intrahepatic duct and abrupt narrowing at distal end. Distal common bile duct joined with pancreatic duct at right angle.
|
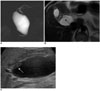 | Fig. 3Choledochal cyst with anomalous union of pancreaticobile duct (Komi IA, Todani IA) combined cholangiocarcinoma.
A-C. MR cholangiopancreatography (A) images show cystic dilatation of common bile duct, with abrupt narrowing at distal end which joined with pancreatic duct at right angle. T2W transverse image of MR cholangiopancreatography (B) and ultrasonography (C) images show polypoid mass (arrows) at proximal region of cyst, which was confirmed pathologically as cholangiocarcinoma after operation.
|
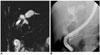 | Fig. 4Choledochal cyst with anomalous union of pancreaticobile duct (Komi IB, Todani IVA) combined stone.
A, B. MR cholangiopancreatography (A) and endoscopic retrograde cholangiopancreatography (B) images show saccular dilatation of intrahepatic duct and common bile duct, which joined with pancreatic duct at right angle. Cyst also showed large filling defect in common bile duct. Common channel is dilated on endoscopic retrograde cholangiopancreatography (B) image.
|
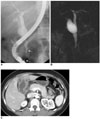 | Fig. 5Choledochal cyst with anomalous union of pancreaticobile duct (Komi IIA, Todani IB) combined small cell cancer of gallbladder.
A-C. Endoscopic retrograde cholangiopancreatography (A) and MR cholangiopancreatography (B) images show fusiform dilatation of common bile duct, joined with pancreatic duct at acute angle, along with long common channel (arrows, 24 mm) and low lying cystic duct insertion with dilatation in proximal region. Irregular filling defect is noted in contrast filled gallbladder on endoscopic retrograde cholangiopancreatography image. CT image (C) shows irregular enhancing wall thickening of gallbladder. Pathologic diagnosis was confirmed to be small cell carcinoma.
|
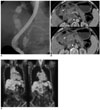 | Fig. 6Choledochal cyst with anomalous union of pancreaticobile duct (Komi IIB, Todani IVA) combined gallbladder and common bile duct cancer.
A-C. Endoscopic retrograde cholangiopancreatography (A) image shows irregularly dilated common bile duct, common hepatic duct and intrahepatic duct. Common bile duct is joined with pancreatic duct at acute angle. In addition, long common channel is dilated. CT (B) image shows irregular enhancing mass in gallbladder (short arrows) and common bile duct (long arrows). PET CT (C) show focal increased FDG uptake as seen in gallbladder and common bile duct region.
|
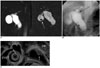 | Fig. 7Choledochal cyst and anomalous union of pancreaticobile duct (Komi IIIC2, Todani IC) combined with gallbladder cancer.
A-C. MR cholangiopancreatography (A) and endoscopic retrograde cholangiopancreatography (B) images show saccular dilatation of common hepatic duct, common bile duct, left intrahepatic duct and cystic duct. Stricture is noted at hilum (black long arrows) on endoscopic retrograde cholangiopancreatography image. There is mass in gallbladder (white arrow) and small filling defect in cyst (short black arrow). Anomalous union of pancreaticobile duct with patent accessory duct is noted and common channel with same caliber of main and accessory duct. On endoscopic US (C), small polypoid mass in choledochal cyst and broad based villous isoechogenic mass in gallbladder. After operation, gallbladder cancer is confirmed, but there is no evidence of mass in choledochal cyst.
|
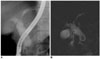 | Fig. 8Choledochal cyst with anomalous union of pancreaticobile duct (Komi IIIC3, Todani IA) combined stone.
A, B. Endoscopic retrograde cholangiopancreatography (A) and MR cholangiopancreatography (B) images show fusiform dilatation of common bile duct, common hepatic duct, and both intrahepatic ducts. Accessory pancreatic duct is united with main pancreatic duct and common channel is dilated. Round filling defect is noted in distal common bile duct.
|
 | Fig. 9Choledochal cyst was described according to Todani's classification system (12). |
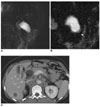 | Fig. 10Choledochal cyst with anomalous union of pancreaticobile duct (Komi IA, Todani IC) combined cholangiocarcinoma and gallbladder cancer with liver metastasis.
A-C. MR cholangiopancreatography images (A, B) show cystic dilatation of common bile duct, however, proximal common hepatic duct and intrahepatic duct show normal caliber. Additionally, common bile duct joined with pancreatic duct at right angle (arrow). CT image (C) shows saccular dilatation of common bile duct with enhancing wall thickening in proximal region of choledochal cyst (arrow) and gallbladder (arrowheads). In addition, metastatic lesions are present in liver and conglomerated lymph nodes at aortocaval and left paraaortic regions.
|
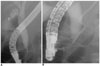 | Fig. 11Choledochal cyst without anomalous union of pancreaticobile duct.
A, B. Endoscopic retrograde cholangiopancreatography images (A, B) show fusiform dilatation of common bile duct. There is normal union of common bile duct and pancreatic duct with less than 12 mm of common channel.
|
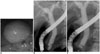 | Fig. 12Choledochocele (Todani III).
A-C. Endoscopic retrograde cholangiopancreatography image revealed that duodenal papilla is bulging. Distal common bile duct shows saccular dilatation and collapsing during procedure.
|
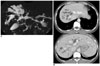 | Fig. 13Calori's disease (Todani V).
A, B. MR cholangiopancreatography (A) image shows multifocal irregular dilatation of intrahepatic duct without common bile duct dilatation. CT (B) image revealed multiple cystic dilatation of intrahepatic duct in both lobes of liver, as well as dot-like enhancing foci representing portal radicles adjacent to dilated intrahepatic duct.
|
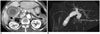 | Fig. 14Choledochal cyst with anomalous union of pancreaticobile duct (Komi IA, Todani IC) combined cholecystitis.
A, B. Initial CT (A) image shows diffuse wall thickening with distension of gallbladder, suggesting acute cholecystitis. MR cholangiopancreatography (B) image shows fusiform dilatation of common bile duct, joined with pancreatic duct at right angle.
|
References
1. Wiseman K, Buczkowski AK, Chung SW, Francoeur J, Schaeffer D, Scudamore CH. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg. 2005. 189:527–531.
2. Liu CL, Fan ST, Lo CM, Lam CM, Poon RT, Wang J. Choledochal cysts in adults. Arch Surg. 2002. 137:465–468.
3. Visser BC, Suh I, Way LW, Kang SM. Congenital choledochal cysts in adults. Arch Surg. 2004. 139:855–862.
4. Liu YB, Wang JW, Devkota KR, Ji ZL, Li JT, Wang XA, et al. Congenital choleochal cysts in adults: twenty-five-year experience. Chin Med J. 2007. 120:1404–1407.
5. Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Lee YS, et al. Can MRCP replace the diagnostic role of ERCP for patients with choledochal cysts? Gastrointest Endosc. 2005. 62:360–366.
6. Sugiyama M, Haradome H, Takahara T, Abe N, Tokuhara M, Masaki T, et al. Anomalous pancreaticobiliary junction shown on multidetector CT. AJR Am J Roentgenol. 2003. 180:173–175.
7. Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing anomalous pancreaticobiliary junction. Gastrointest Endosc. 1997. 45:261–267.
8. Babbitt DP, Starshak RJ, Clemett AR. Choledochal cysts: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med. 1973. 119:57–62.
9. Iwai N, Yanagihara J, Tokiwa K, Shimotake T, Nakamura K. Congenital choledochal dilatation with emphasis on pathophysiology of the biliary tract. Ann Surg. 1992. 215:27–30.
10. Metcalfe MS, Wemyss-Holden SA, Maddern GJ. Management dilemmas with choledochal cysts. Arch Surg. 2003. 138:333–339.
11. Komi N, Takehara H, Kunitomo K, Miyoshi Y, Yagi T. Does the type of anomalous arrangement of pancreaticobiliary ducts influence the surgery and prognosis of choledochal cyst? J Pediat Surg. 1992. 27:728–731.
12. Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1997. 134:263–269.
13. Todani T, Watanabe Y, Toki A, Morotomi Y. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat Surg. 2003. 10:340–344.
14. Fieber SS, Nance FC. Choledochal cyst and neoplasm: a comprehensive review of 106 cases and presentation of two original cases. Am Surg. 1997. 63:982–987.
15. Elnemr A, Ohta T, Kayahara M, Kitagawa H, Yoshimoto K, Tani T, et al. Anomalous pancreaticobiliary ductal junction without bile duct dilatation in gallbladder cancer. Hepatogastroenterology. 2001. 48:382–386.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download