Abstract
Primary gastric carcinoma is the most common cause of linitis plastica. Less frequently, metastatic gastric cancer from the breast, omental metastases and non-Hodgkin lymphoma involving the stomach have been reported to show similar radiographic findings as for linitis plastica. A metastatic gastric cancer from bladder cancer is extremely rare. We present an unusual case, the first to our knowledge, of gastric linitis plastica that resulted from a metastatic urothelial carcinoma of the bladder.
Gastric linitis plastica is a classical feature of a scirrhous gastric carcinoma on barium studies characterized by irregular narrowing and rigidity of the stomach (1). Metastases to the stomach are unusual but have been occasionally reported in patients with malignancies. Most of the metastases arise via a hematogenous route from malignant melanoma and carcinoma of the breast or lung, Much less frequently, a thyroid, testicular, ovarian or pancreas carcinoma can metastasize to the stomach (1-3). Metastatic gastric cancer from bladder cancer is extremely rare. To the best of our knowledge, although Wallmeroth et al. (4) have reported six cases of gastric metastases in 367 patients with bladder cancer and Pak et al. (5) have reported one case with multiple metastases including the stomach, both investigations were autopsy studies and there have been no reports in the imaging literature (4, 5). Menuck and Amberg (3) described the radiographic patterns of metastatic gastric cancer as (a) the presence of a solitary polypoid submucosal mass, (b) the presence of multiple polypoid submucosal masses and (c) an infiltrating constricting pattern similar to 'linitis plastica' (3). Carcinoma of the breast is well known to metastasize in an infiltrative 'linitis plastica' pattern, which is an uncommon pattern for metastases from other malignancies (3, 6, 7). We report here a case of metastatic gastric cancer from the bladder, which presented as linitis plastica mimicking a primary gastric cancer.
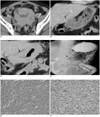
A 60-year-old man visited the emergency room with projectile vomiting that had persisted for five days. There was a palpable mass located in the epigastric area. Seven months earlier, the patient presented with hematuria and was diagnosed with bladder cancer (Fig. 1A). No definite abnormality in the stomach was seen on an initial image (Fig. 1B). A pathological examination obtained from the initial diagnosis reported a poorly differentiated infiltrating urothelial carcinoma as a clear cell variant. Subsequently, the patient had undergone systemic chemotherapy and locoregional radiation therapy for bladder cancer. Due to the new symptoms, an abdominal computed tomography scan was performed with the use of a 64-channel multidetector CT (LightSpeed VCT; GE Healthcare, Waukesha, WI) scanner for the precontrast phase and portal phase after the administration of an intravenous iodinated contrast agent (Ultravist; Bayer Schering Pharma, Berlin, Germany). Axial and coronal reformatted images were obtained with a 5 mm thickness. The primary bladder tumor mass had markedly regressed in extent and size with residual tumor infiltration to the right pelvic side wall. The stomach showed diffuse and marked gastric wall thickening from the gastric cardia to the antrum (Fig. 1C). Subsequently, gastroduodenoscopy was performed and there was an approximate 8 cm segmental infiltrative submucosal lesion resulting from luminal narrowing from the lower body to the pyloric ring. There was no ulcerative lesion in the involved mucosa. An endoscopic biopsy was performed, but only inflammatory neutrophils were seen. An upper gastrointestinal (GI) series was performed that revealed marked mucosal thickening from the gastric lower body to the pyloric antrum. There was abrupt narrowing in the gastric antrum with rigidity and loss of peristalsis, producing a linitis plastica appearance (Fig. 1D). To relieve symptoms and to provide pathological confirmation, a feeding jejunostomy with a loop ileostomy and multiple biopsies were performed. On the operative field, multiple nodular lesions were seen in the stomach with direct invasion to the transverse colon. In addition, multiple seeding masses were seen in the ileum, greater omentum and mesentery. Multiple biopsies were performed for the stomach, transverse colon and multiple seeding masses located in the ileum, greater omentum and mesentery. The biopsy specimens of the stomach and the seeding masses were infiltrated by numerous poorly differentiated malignant cells with eccentric nuclei and moderate to abundant cytoplasm (seen at a magnification of ×200) (Fig. 1E). Immunohistochemical analysis showed positive staining for cytokeratin (CK) 7, CK20, high-molecular-weight cytokeratin (CK34BE12) and p63 (Fig. 1F) and negative staining for CK20. The stomach tissue was pathologically confirmed as being poorly differentiated, with the presence of an infiltrating metastatic urothelial carcinoma confirmed by the use of special stains and immunohistochemical studies.
Bladder cancer is the fifth most common malignancy in males of Western countries. Wallmeroth et al. (4) have reported that the regional lymph nodes, lung, liver and bone are the most frequent sites of metastases. These investigators also reported six cases of gastric metastases in 367 patients with bladder cancer based on autopsy findings. Additionally, Pak et al. (5) reported one autopsy case but there has been no report of stomach involvement in the imaging literature.
Gastric metastases most frequently occur from malignant melanoma, followed by carcinoma of the breast or lung mainly via a hematogenous route. Less frequently, lymphatic spread of a tumor or direct extension from the neighboring structures can be a route of metastases. Hematogenous metastatic lesions usually implant in the submucosal layer of the stomach, appearing as one or multiple discrete submucosal nodules that may ulcerate and the metastatic lesions are sometimes manifested by larger masses. Another unusual form is linitis plastica or 'leather bottle' appearance, mostly caused by the infiltrating lobular form of breast carcinoma.
Metastatic linitis plastica can also form by several routes, including hematogenous metastases, lymphatic spread and direct extension. Metastatic linitis plastica is indistinguishable from that of a primary scirrhous carcinoma of the stomach. Although rare, infiltrating lobular carcinoma of the breast has been reported as the most common metastatic cause of gastric linitis plastica (6, 7). Recently, the malignant target sign has been described as a feature of metastatic linitis plastica by Ha et al. (8) and has been emphasized by Gollub et al. (9). The tumor infiltrates and expands the mucosa, submucosa and serosa much more as compared to the muscularis propria, resulting in a less thickened and hypoattenuated middle zone of the target sign. However, this feature is seen most typically in the rectum or other parts of the colon and occasionally in other parts of the gastrointestinal tract (8, 9). In our case, the entire gastric wall was thickened with slightly heterogeneous enhancement and the malignant target sign was not seen. With underlying scirrhous-type primary bladder cancer, this finding should be considered as suspicious for a metastasis rather than for a primary gastric cancer. As the primary bladder mass had markedly regressed after systemic chemotherapy and locoregional radiation therapy, the presence of a metastasis at an unusual site might be in doubt. However, residual tumor infiltrations in the right pelvic side wall and newly noted multiple peritoneal seeding nodules represent a partial regression state of the tumor, which could metastasize.
The histological distinction between a primary gastric carcinoma and metastatic urothelial carcinoma is difficult in poorly differentiated lesions as in our case, and the use of confirmatory immunohistochemical stains is necessary to establish the origin of the tumor. A variety of markers such as CK7, CK20, CK34BE12 and p63 have been used as potential urothelial markers. These markers are not entirely specific for urothelial carcinoma but are helpful when used in combination. Among these markers, p63 is known to be a more sensitive and specific marker for urothelial carcinoma and the use of p63 is more helpful when the protein is co-expressed with other markers (10).
This case is of interest for several reasons. First, despite an advanced stage, it is clinically important to determine whether gastric linitis plastica is primary or metastatic. This information is necessary not only to make a decision for the chemotherapy regimen but also whether to perform surgery considered after neoadjuvant chemotherapy in the case of a primary gastric cancer. Unfortunately, an endoscopic biopsy may be negative in up to 30% of gastric linitis plastica cases; thus, differentiation is difficult and important. Second, although a metastasis to the stomach from a primary cancer is rare, such as for advanced bladder cancer in this case, the possibility of a metastasis to the stomach should be considered whenever there is an underlying malignancy. Third, one should be aware that bladder cancer could metastasize as linitis plastica to the stomach, although most cases of linitis plastica are caused by primary gastric carcinoma, metastatic breast cancer, omental metastases and non-Hodgkin lymphoma. To the best of our knowledge, this is the first report of an infiltrating urothelial carcinoma of bladder presenting as linitis plastica of the stomach.
Figures and Tables
Fig. 1
Imaging findings are presented for 60-year-old man with underlying bladder cancer presenting as linitis plastica.
A. Axial enhanced CT image obtained seven months earlier shows massive bladder wall thickening with slightly heterogeneous enhancement (solid arrows), which was diagnosed as bladder cancer. Bladder mass invaded seminal vesicle, prostate and adjacent pelvic walls (T4).
B. 5 mm thick, reformatted coronal image obtained seven months earlier shows no evidence of abnormal wall thickening or focal lesion in stomach.
C. 5 mm thick, reformatted coronal image obtained on recent visit to emergency room shows newly noted diffuse gastric wall thickening with dense enhancement and marked perigastric infiltrations (solid arrows). Multiple nodular lesions are noted along transverse mesocolon with diffuse segmental wall thickening in mid portion of transverse colon (arrowheads), suggesting transverse colon invasion.
D. Upper GI series shows marked gastric mucosal thickening from lower body to pyloric antrum of stomach (solid arrows). There was abrupt narrowing at gastric antrum with rigidity and loss of peristalsis (arrowhead), producing appearance of linitis plastica.
E. Photomicrograph (Hematoxylin & Eosin staining, ×200) of gastric biopsy specimen shows numerous poorly differentiated malignant cells with eccentric nuclei and moderate to abundant cytoplasm.
F. Immunohistochemical analysis shows positive staining for p63.
References
1. Gore RM, Levine MS. Textbook of gastrointestinal radiology. 2008. 3rd ed. Philadelphia: Saunders;628–649.
2. Kim SY, Kim KW, Kim AY, Ha HK, Kim JS, Park SH, et al. Bloodborne metastatic tumors to the gastrointestinal tract: CT findings with clinicopathologic correlation. AJR Am J Roentgenol. 2006. 186:1618–1626.
3. Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975. 20:903–913.
4. Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): an autopsy study on 367 patients. Urol Int. 1999. 62:69–75.
5. Pak K, Ishida A, Arai Y, Tomoyoshi T. An autopsy case of untreated bladder cancer. Hinyokika Kiyo. 1987. 33:1261–1265.
6. Kanne JP, Mankoff DA, Baird GS, Minoshima S, Livingston RB. Gastric linitis plastica from metastatic breast carcinoma: FDG and FES PET appearances. AJR Am J Roentgenol. 2007. 188:W503–W505.
7. Levine MS, Kong V, Rubesin SE, Laufer I, Herlinger H. Schirrous carcinoma of the stomach: radiologic and endoscopic diagnosis. Radiology. 1990. 175:151–154.
8. Ha HK, Jee KR, Yu E, Yu CS, Rha SE, Lee IJ, et al. CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. AJR Am J Roentgenol. 2000. 174:463–466.
9. Gollub MJ, Schwartz MB, Shia J. Scirrhous metastases to the gastrointestinal tract at CT: the malignant target sign. AJR Am J Roentgenol. 2009. 192:936–940.
10. Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol. 2006. 125:675–681.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download