Diffuse esophageal leiomyomatosis is a rare benign disease of proliferating smooth muscle that can be accompanied by leiomyoma of extraesophageal organs including the tracheobronchial and genital tracts (1-3). Moreover, diffuse leiomyomatosis can be associated with Alport syndrome in familial cases (2, 4). 18F-fluoroseoxyglucose positron emission tomography (18F-FDG-PET) can be useful in diagnosis since the benign masses can form lesions in the esophagus and extraesophageal organs that may be confused with malignancy. However, few reports have been published on the use of 18F-FDG PET in diffuse leiomyomatosis involving multiple organs. We describe 18F-FDG PET/computed tomography (CT) findings in a patient with esophageal and genital leiomyomatosis.
CASE REPORT
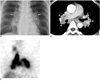
A 49-year-old woman was hospitalized for further evaluation of dysphagia. Esophagoscopy revealed a huge mass in the lower esophageal portion. Abdominal CT examination demonstrated a huge low-attenuated submucosal mass in the esophagus. Additional heterogeneous highly-attenuated masses were evident in the uterus and vulvar region. While diagnosis of gastrointestinal stromal tumor or leiomyoma is typical from such CT observations, preferred according to conventional CT, a malignancy cannot be fully excluded. Appropriately, the patient was referred to the nuclear medicine department for 18F-FDG PET/CT to differentiate the esophageal and genital masses as malignant or benign. After a 6 hours fast, the patient was intravenously administered 370 MBq 18F-FDG. At the time of injection, serum glucose level was 97 mg/dl. The patient was instructed to rest comfortably for 60 min while whole body PET/CT images were obtained using a Gemini Dual PET/CT scanner (Philips Medical Systems, Amsterdam, The Netherlands). Seven frames (2.5 min/frame) of emission PET data were acquired in a three-dimensional (3D) mode after non-contrast CT scans from the base of the skull to the upper thigh (tube rotation time of 1 second per revolution, 120 kV, 50 mA, 6.5 mm per rotation, and acquisition time of 43.52 second for a scan length of 856.5 mm). Emission PET images were reconstructed with non-contrast CT using 3D-Row-Action Maximum-Likelihood Algorithm reconstruction (field of view = 576 mm, slice thickness = 4 mm, matrix size = 144×144, voxel size = 4.5×4.5×4 mm). Standardized uptake value (SUV) was calculated based on the injected dose and patient body weight. PET/CT demonstrated intensely increased 18F-FDG uptake with 3.8 of maximal SUV in the esophagus, 6.13 in the uterus, and 2.81 in the vulvar mass (Fig. 1A-D), which was indicative of high probabilities of malignancy. Each mass was excised. Pathological testing confirmed that each mass was benign leiomyomatosis (Fig. 1E-J). The patient's son was remarkable for Alport syndrome, and had undergone removal of an esophageal leiomyomatosis nine years previously. However, the patient showed no specific abnormal sign of Alport syndrome.
DISCUSSION
Leiomyoma in the esophagus must be differentiated from malignant esophageal carcinoma, cyst, or bulbous stricture. Rarely, thickened layers of the esophageal wall can also be caused by diffuse leiomyomatosis, a benign muscular abnormality (1). Diffuse leiomyomatosis is also known as giant muscular hypertrophy with diffuse proliferation of the esophageal smooth muscle. The large tumor formation predominantly occurs in the middle and distal third of the esophagus. The most common clinical symptoms are dysphasia, vomiting, and retrosternal pain due to luminal narrowing and motor disorder of esophagus. These leiomyomatosis frequently involve extraesophageal organs such as tracheobronchial tree or female genital tract (uterus, vagina, vulva, and clitoris) and also show hyperplasia of the thoracic or genital smooth muscles (2, 3). The familiar form of leiomyomatosis can be associated with Alport syndrome, a hereditary disease that displays hematuric nephropathy and sensorial deficiencies including sensorineural hearing loss and congenital cataracts. It is transmitted as a X-linked dominant and may affect females without sign of nephropathy, which is revealed as Alport syndrome carrier status (4-7). This was presently the case. Previous reports have described increased 18F-FDG uptake in leiomyomas (8-11). Leiomyoma with intense 18F-FDG accumulation can be confused with a malignant lesion. The cause of hypermetabolism of this benign condition is probably due to higher levels of growth factors and increased peristaltic activity in smooth muscles (3, 8-11). Approximately 10% of leiomyomas in pre-manopausal women display focal 18F-FDG uptake (maximal SUV > 3.0) (12). In fact, malignant tumors cannot be distinguished from hypermetabolic benign leiomyomas using 18F-FDG PET. Thus, leiomyomatosis of multiple organs may be a cause of false-positive PET and represents a pitfall for correct diagnosis between benign and malignant lesions.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download