Abstract
Objective
This study was designed to determine prospectively the expression of the multifunctional CD98 protein in peripheral white blood cells in patients receiving iodinated contrast media (CM) for a computed tomography (CT) examination.
Materials and Methods
In 12 adult patients that received non-ionic dimeric CM (iosimenol or iodixanol), the expression of CD98 was analyzed from samples of peripheral white blood cells obtained prior to, one hour, and 24 hours after CM injection by the use of flow cytometry analysis and the use of the direct immunofluorescence technique.
Results
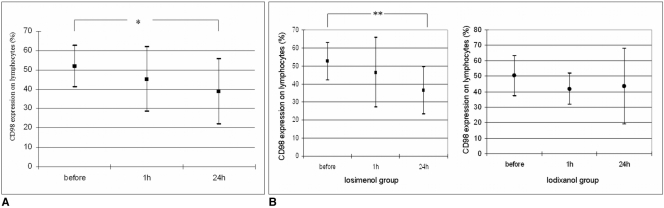
Overall, expression of CD98 was significantly downregulated 24 hours after CM injection (51.9%±10.8% vs. 38.8%±16.9%; p < 0.04). Patients that received iosimenol exhibited a more pronounced but not significant decrease of CD98 expression both one hour and 24 hours after CM injection. In an analysis of specific patient responses, CD98 downregulation occurred in eight patients. In two patients, CD98 was upregulated, and in the remaining two patients, expression remained unchanged. No patient acquired an adverse CM reaction.
Conclusion
This is the first demonstration that CM may be a regulator of CD98 expression. To determine if upregulation is associated with an increased risk for the acquisition of an adverse CM-induced hypersensitivity reaction and if downregulation is associated without a risk for the acquisition of an adverse CM-induced hypersensitivity reaction, further studies with a larger population of patients are required.
Although the use of iodinated X-ray contrast media (CM) has been shown to be safe in patients, the use of CM can induce hypersensitivity reactions in a small percentage of patients that may occur immediately or may be delayed (1-8). Immediate hypersensitivity reactions, whether allergic or non-allergic in nature, are mediated by biogenic amines such as histamine and tryptase that are released by basophils and/or mast cells and are clinically characterized by anaphylaxis (urticaria, angioedema, laryngeal edema, bronchial constriction and occasionally cardiac arrest and loss of awareness). On the contrary, both non-immediate drug reactions in general and CM reactions in particular are primarily T-cell hypersensitivity reactions (1, 9-11) that are immunohistologically detectable in patients with such manifestations (12, 13). Histopathological findings are nonspecific and cannot be distinguished from other drug reactions (13). Therefore, T-cell reactivity has been assessed by proliferation in response to the drug with use of the lymphocyte transformation test (9, 10) or by the ability to enhance the expression of activation molecules such as CD69 (9-11).
The heterodimeric L-type amino acid transporter 1 (LAT1/CD98), initially described as early T-cell activation marker, has been shown to exert multiple cell functions including cell adhesion and cell fusion (14-18). Moreover, LAT1/CD98 vectorially transports both drugs (e.g. pregabalin) and 3-iodo-α-methyl-L-tyrosine across the cell membrane into the cell (19-21). It is currently questionable if iodinated CM that are intravenously injected for enhanced computed tomography (CT) are able to influence the expression of CD98 in circulating white blood cells. Therefore, the goal of this study was to determine the expression of the CD98 protein in peripheral blood cells in patients that received two different non-ionic dimeric contrast agents and to determine if CD98 expression was upregulated or downregulated.
This study was performed in 12 adult patients (males, n = 8) who underwent diagnostic CT examination. All subjects participated in a clinical double-blinded trial that evaluated the frequency and type of CM-induced side effects by comparing the use of non-ionic dimeric CM iosimenol and iodixanol. The local ethics committee approved the study. Patients provided informed consent for the study and for blood/serum sampling. Adult patients were included (i) that were not receiving immunosuppressive drugs (e.g. glucocorticosteroids, azathioprine, cyclophosphamide) or antihistamines, (ii) who had undergone their last therapeutic radiation therapy and/or chemotherapeutic cycle more than six weeks prior and (iii) who had no history of previous CM side effects. The mean patient age was 55.3 years (age range, 45-64 years). Blood samples were collected prior to, one hour and 24 hours after CM injection by the use of sterile venipuncture.
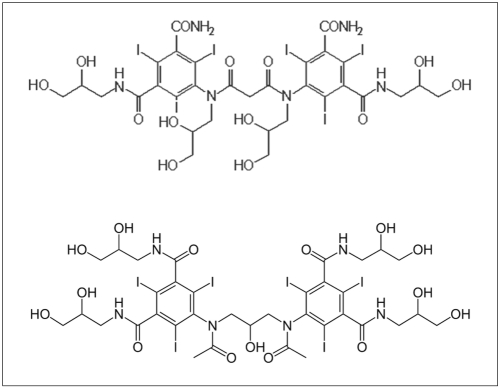
Dimeric iodixanol (Visipaque; GE Healthcare, Freiburg, Germany) and dimeric iosimenol (Interpharma, Prague, Czech Republic) were supplied as sterile, aqueous solutions of 270 mg I/mL (osmolality of -300 mosm/kg) or 280 mg I/mL (osmolality of -300 mosm/kg), respectively. The chemical structures of both compounds are shown in Figure 1. The CM were injected intravenously at a rate of 2 ml/sec. The patients were administered 100 ml of either iodixanol or iosimenol.
Peripheral blood mononuclear cells (PBMC) were separated by the use of Ficoll-Hypaque (Biochrom, Berlin, Germany) density gradient centrifugation and were stained with direct phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)- or peridinin chlorophyll protein (PerCP)- conjugated mAbs. Appropriate isotype control antibodies and anti-CD3 (mouse IgG1, clone SK7), anti-CD4 (mouse IgG1, clone SK3), anti-CD8 (mouse IgG1, clone SK1), anti-CD45 (mouse IgG1, clone 2D1), anti-HLA-DR (mouse IgG1, clone L243), anti-CD14 (mouse IgG2a, clone TUK4) and anti-CD98 (mouse IgG1, clone 4F2) were purchased from Becton-Dickinson (Mountain View, CA). For each sample, 10,000 events were acquired, the data were stored and then analyzed by the use of FACScan Research software (CellQuest, Becton Dickinson). Immunofluorescence analysis was performed by the use of dot plot and histogram analysis. Values were expressed as the percentage of the gated total lymphocyte population.
Data are expressed as mean±standard deviation. The normal distribution of the investigated subsets was demonstrated by use of the Kolmogorov-Smirnov test. Significance of differences between means was calculated using the Student's t-test for paired and unpaired data. Statistical analysis of different groups was conducted using the ANOVA-test with Bonferroni correction for multiple comparisons. The Chi-squared test was used to test the differences in the frequency of adverse reactions in patients that received iosimenol and iodixanol. Differences between values were considered statistically significant at p < 0.05.
In patients that received iosimenol, a decrease of the CD98 expression was detected both after one hour and after 24 hours (Fig. 3). The decrease in expression after 24 hours was more pronounced than after one hour. In patients that received iodixanol, CD98 expression initially decreased after one hour and then increased (Fig. 3). The differences between CD98 expression in patients that received either iosimenol or iodixanol were not statistically different.
In most patients (n = 8, 5 males) CM Injection induced downregulation of CD98 expression. In two patients that received iodixanol (1 male and 1 female), upregulation of CD98 occurred and in two other patients (2 males) CM injection did not induce any CD98 expression change.
To determine if upregulation or downregulation might correspond to an allergic reaction cannot be answered in the present investigation as none of patients acquired an unwanted side effect. In a large patient study population (80 patients; 65 males), 10% of patients that received iosimenol (males:females = 3:1) and 8% of patients (all males) that received iodixanol acquired non-immediate reactions (data not shown). This difference was not statistically significant (p > 0.06).
The goal of the present study was to determine if injection of dimeric non-ionic contrast agents (iosimenol and iodixanol) under routine conditions in patients undergoing a CT examination is able to affect the expression of the T-cell activation molecule CD98 in PBMC. We found that dimeric CM injection has the pharmacological ability to induce either upregulation or downregulation of CD98.
CD98 (also known as 4F2 antigen and 125 kDa N-glycosylated type II transmembrane glycoprotein) is the heavy chain of the heterodimeric amino acid transport system (HAT) that is expressed in almost all cell types except platelets (15-18, 22-25). The HAT is composed of two polypeptides: a heavy subunit that is covalently associated with one of different light chains (also called LAT1, LAT2). The light chain is the transporter and the heavy chain is required for the functional cell surface expression and localization of the HAT complex (25). CD98 initially identified as an activation marker molecule has diverse cellular functions such as amino acid transport, cell adhesion (i.e. integrin beta1 function), migration and perhaps polarization (17, 22-25), and is implicated in the regulation of cellular differentiation, adhesion, growth and apoptosis (15).
Previously, it has been shown that IFN-gamma mediates the transcriptional regulation of CD98 (23), but its regulation seems to be independent of the interleukin 2/interleukin 2 receptor system (14). Upregulation of CD98 expression also occurs during lymphocyte activation induced by mitogens, superantigens, conventional antigens and a combination of phorbol myristate acetate and ionomycin (14). Several substances inhibit the expression of CD98 such as rolipam, a potent and selective phosphodiesterase inhibitor (26). Similar results have been obtained with the use of pentoxifylline. This agent has a significant inhibitory effect on the T lymphocyte expression of the activation antigens CD25 (IL-2R alpha-chain), CD69 (activation-inducer molecule), and CD98 (4F2) induced by phytohaemagglutinin (27). Dimeric contrast agents seem either to inhibit or to enhance the expression of CD98. Since both injected contrast agents are of blood iso-osmolality, this physico-chemical property did not seem responsible for the observed changes in CD98 expression. Although both compounds are dimeric non-ionic contrast agents of a similar chemical structure (Fig. 1), they induced different CD98 expression patterns one hour and 24 hours after CM injection (Fig. 3B). Moreover, it was remarkable that dimeric non-ionic CM administration can either upregulate or downregulate the CD98 cell surface receptor. In addition, in some patients, expression of CD98 did not change after intravenous CM injection.
Interestingly, expression of CD98 is increased in allergic patients (28). The increased risk for these patients to acquire an adverse CM-induced reaction might be a result of increased CD98 expression. Although from the preset study it is not possible to draw general conclusions, it is tempting to speculate that downregulation of CD98 expression that occurred in most patients might be associated with no risk for hypersensitivity while upregulation of this activation marker could be a sign for an increased risk for the acquisition of such a reaction.
The transport-related proteins BAT and 4F2hc are not specific for amino acids and can mediate the cross membrane transport of a variety of other agents such as uridine, pyruvate, thyroid hormone and drugs such as pregabalin, and melphalan (20, 21, 24, 29, 30). From the present study it is not possible to determine if the CD98 protein mediates the transport of iodinated CM. However, both the thyroid hormone and 3-iodo-α-methyl-L-tyrosine transport is mediated by heterodimers of the 4F2 heavy-chain (19, 20) and it is well established that traces of free iodine present in iodinated contrast agents solutions interfere with the physiological iodine thyroid uptake competitively in the thyroid hormone transport system.
The main limitation of this study is that no patient exhibited a hypersensitivity reaction. Therefore, it would be necessary to investigate this activation marker in patients who have a positive history of a CM-related non-immediate hypersensitivity. Another limitation of the presented trial is the lack of a control population that received saline injection instead of the contrast agent to exclude other factors that could influence CD98 regulation. The study environment or the injection procedure could theoretically induce modification of CD98 expression. Therefore, in future studies, additional control subjects need to be employed.
In conclusion, we have provided for the first time evidence that CM injection might influence the expression of the CD98 protein. The current study is a preliminary proof of concept. CD98 is an immunological activation marker and if CM-induced upregulation of CD98 expression contributes to adverse hypersensitivity reactions is still unclear. To address this question in more detail, an investigation with a larger patient population is warranted.
References
1. Webb JA, Stacul F, Thomsen HS, Morcos SK. Members of the Contrast Media Safety Committee of the European Society of Urogenital Radiology. Late adverse reactions to intravascular iodinated contrast media. Eur Radiol. 2003; 13:181–184. PMID: 12541128.
2. Christiansen C, Pichler WJ, Skotland T. Delayed allergy-like reactions to X-ray contrast media: mechanistic considerations. Eur Radiol. 2000; 10:1965–1975. PMID: 11305580.

3. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990; 175:621–628. PMID: 2343107.

4. Delayed allergy-like reactions to X-ray contrast media. Exploration of the problem. Eur Radiol. 1996; 6:1–24. PMID: 9403083.
5. Schild HH, Kuhl CK, Hübner-Steiner U, Böhm I, Speck U. Adverse events after unenhanced and monomeric and dimeric contrast-enhanced CT: a prospective randomized controlled trial. Radiology. 2006; 240:56–64. PMID: 16720865.

6. Mikkonen R, Kontkanen T, Kivisaari L. Acute and late adverse reactions to low-osmolal contrast media. Acta Radiol. 1995; 36:72–76. PMID: 7833173.

7. Böhm I, Schild HH. A practical guide to diagnose lesser-known immediate and delayed contrast media-induced adverse cutaneous reactions. Eur Radiol. 2006; 16:1570–1579. PMID: 16770655.

8. Lapi F, Cecchi E, Pedone C, Attanasio F, Banchelli G, Vannacci A, et al. Safety aspects of iodinated contrast media related to their physicochemical properties: a pharmacoepidemiology study in two Tuscany hospitals. Eur J Clin Pharmacol. 2008; 64:723–737. PMID: 18401577.

9. Beeler A, Zaccaria L, Kawabata T, Gerber BO, Pichler WJ. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008; 63:181–188. PMID: 18005225.

10. Torres MJ, Mayorga C, Cornejo-Garcia JA, Lopez S, Chaves P, Rondon C, et al. Monitoring non-immediate allergic reactions to iodine contrast media. Clin Exp Immunol. 2008; 152:233–238. PMID: 18341616.

11. Lerch M, Keller M, Britschgi M, Kanny G, Tache V, Schmid DA, et al. Cross-reactivity patterns of T cells specific for iodinated contrast media. J Allergy Clin Immunol. 2007; 119:1529–1536. PMID: 17412404.

12. Kanny G, Pichler W, Morisset M, Franck P, Marie B, Kohler C, et al. T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005; 115:179–185. PMID: 15637566.

13. Delgado-Jimenez Y, Perez-Gala S, Aragüés M, Sanchez-Perez J, Garcia-Diez A. Late skin reaction to iodixanol (Visipaque): clinical manifestations, patch test study, and histopathological evaluation. Contact Dermatitis. 2006; 55:348–353. PMID: 17101010.

14. Komada H, Imai A, Hattori E, Ito M, Tsumura H, Onoda T, et al. Possible activation of murine T lymphocyte through CD98 is independent of interleukin 2/interleukin 2 receptor system. Biomed Res. 2006; 27:61–67. PMID: 16707844.

15. Cho JY, Skubitz KM, Katz DR, Chain BM. CD98-dependent homotypic aggregation is associated with translocation of protein kinase Cdelta and activation of mitogen-activated protein kinases. Exp Cell Res. 2003; 286:1–11. PMID: 12729789.
16. Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, et al. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood. 2001; 98:374–382. PMID: 11435306.
17. Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, et al. CD98 modulates integrin beta1 function in polarized epithelial cells. J Cell Sci. 2005; 118:889–899. PMID: 15713750.
18. Suga K, Katagiri K, Kinashi T, Harazaki M, Iizuka T, Hattori M, et al. CD98 induces LFA-1-mediated cell adhesion in lymphoid cells via activation of Rap1. FEBS Lett. 2001; 489:249–253. PMID: 11165259.

19. Shikano N, Kanai Y, Kawai K, Ishikawa N, Endou H. Characterization of 3-[125I]iodo-alpha-methyl-L-tyrosine transport via human L-type amino acid transporter 1. Nucl Med Biol. 2003; 30:31–37. PMID: 12493540.
20. Friesema EC, Docter R, Moerings EP, Verrey F, Krenning EP, Hennemann G, et al. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology. 2001; 142:4339–4348. PMID: 11564694.

21. Su TZ, Feng MR, Weber ML. Mediation of highly concentrative uptake of pregabalin by L-type amino acid transport in Chinese hamster ovary and Caco-2 cells. J Pharmacol Exp Ther. 2005; 313:1406–1415. PMID: 15769862.

22. Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002; 61:729–737. PMID: 11901210.

23. Yan Y, Dalmasso G, Sitaraman S, Merlin D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G535–G545. PMID: 17023546.
24. Yao SY, Muzyka WR, Cass CE, Cheeseman CI, Young JD. Evidence that the transport-related proteins BAT and 4F2hc are not specific for amino acids: induction of Na+-dependent uridine and pyruvate transport activity by recombinant BAT and 4F2hc expressed in Xenopus oocytes. Biochem Cell Biol. 1998; 76:859–865. PMID: 10353721.

25. Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, et al. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999; 274:3009–3016. PMID: 9915839.

26. Layseca-Espinosa E, Baranda L, Alvarado-Sanchez B, Portales-Perez D, Portillo-Salazar H, Gonzalez-Amaro R. Rolipram inhibits polarization and migration of human T lymphocytes. J Invest Dermatol. 2003; 121:81–87. PMID: 12839567.

27. Gonzalez-Amaro R, Portales-Perez D, Baranda L, Redondo JM, Martinez-Martinez S, Yanez-Mo M, et al. Pentoxifylline inhibits adhesion and activation of human T lymphocytes. J Immunol. 1998; 161:65–72. PMID: 9647208.
28. Taylor ML, Noble PW, White B, Wise R, Liu MC, Bochner BS. Extensive surface phenotyping of alveolar macrophages in interstitial lung disease. Clin Immunol. 2000; 94:33–41. PMID: 10607488.

29. Harada N, Nagasaki A, Hata H, Matsuzaki H, Matsuno F, Mitsuya H. Down-regulation of CD98 in melphalan-resistant myeloma cells with reduced drug uptake. Acta Haematol. 2000; 103:144–151. PMID: 10940652.

30. Ritchie JW, Peter GJ, Shi YB, Taylor PM. Thyroid hormone transport by 4F2hc-IU12 heterodimers expressed in Xenopus oocytes. J Endocrinol. 1999; 163:R5–R9. PMID: 10556789.

Fig. 2
Representative fluorescence microscopy of peripheral white blood cells expressing CD98 after staining with monoclonal anti-CD98 antibody labelled with FITC (fluorescein isothiocyanate).

Fig. 3
Modulation of the CD98 expression under the influence of contrast media injection.
A. Contrast media injection in patients that received iosimenol and iodixanol induced non-significant decrease of CD98 expression after one hour and significant decrease of CD98 expression in peripheral lymphocytes after 24 hours (*p < 0.04).
B. CD98 expression in lymphocytes (%) ± standard deviation prior to and at one hour and at 24 hours after injection of either iosimenol (left diagram) or iodixanol (right diagram). Decrease of CD98 expression in patients that received iosimenol was significant after 24 hours (**p < 0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download