This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To evaluate whether the removal of an intraductal mass using an ultrasound (US)-guided directional vacuum-assisted device can eliminate symptoms in patients presenting with abnormal nipple discharge.
Materials and Methods
Between March 2004 and October 2006, 36 patients who presented with abnormal nipple discharge, underwent US-guided, 11-gauge vacuum-assisted biopsy for a benign intraductal single mass on US. The ability of the procedure to eliminate nipple discharge was evaluated by physical examination during follow-up US. Lesion characteristics, biopsy variables, and histologic features were analyzed to identify factors affecting symptom resolution.
Results
Of the 36 lesions, 25 (69%) were intraductal papillomas, 10 (28%) were fibrocystic changes, and one (3%) was a fibroadenoma. The nipple discharge disappeared in 69% (25 of 36) of the women at a mean follow-up time of 25 months (range 12-42 month). There was no difference in the lesion characteristics, biopsy variables, and the histologic features between groups that eliminated the symptom compared those with persistent nipple discharge.
Conclusion
US-guided directional vacuum-assisted removal of an intraductal mass appears to eliminate nipple discharge in only 69% of patients and thus, it should not be considered as an alternative to surgical excision.
Go to :

Keywords: Nipple discharge, Intraductal papilloma, Ultrasound guided, Vacuum-assisted device
Abnormal nipple discharge is defined as unilateral, single-pore, spontaneous discharge. The standard treatment for abnormal nipple discharge is surgical excision, which has been reduced from a complete mastectomy to a single duct excision, because it is known that 80-90% of nipple discharge cases are caused by benign diseases (e.g., benign papilloma, fibrocystic change, or ductectasia), and that only 10-15% of cases are caused by breast carcinomas (
1,
2).
The identification and localization of the cause of nipple discharge is the key to successful treatment (
3). Although a galactography has been preferentially used for the identification and localization of the cause of nipple discharge, recent advances in high-resolution breast ultrasonography (US) or MR galactography allow retro-areolar ducts and small solid masses within these ducts to be clearly demonstrated (
4). Several researchers have reported that performing an US to detect intraductal abnormalities in patients with an abnormal nipple discharge is comparable to that of a galactography, and further, have suggested that US can be used to complement or alternative to a galactography (
5,
6).
During the last decade, percutaneous image-guided needle biopsies have allowed for correct diagnosis of breast lesions. Moreover, the removal of lesions by an image-guided directional vacuum-assisted biopsy has been found to reduce sampling errors, radiologic-histological discordances, rebiopsies, and histologic underestimations, which are the main limitations of percutaneous needle biopsies (
7,
8). Furthermore, the incidental treatment of nipple discharge during US-guided directional vacuum-assisted removal (US-DVAR) has been reported to have success rates ranging from 95 to 97% (
9-
11). However, these studies only included intraductal papillomas (
10) and have lacked long-term follow-up information (
11). Relatively few studies have addressed the possibility of using US-guided vacuum-assisted removal for symptom elimination in patients presenting with abnormal nipple discharge.
Thus, the purpose of this study was to evaluate whether the removal of an intraductal mass using an US-guided vacuum-assisted device can eliminate the symptoms in patients presenting with abnormal nipple discharge.
MATERIALS AND METHODS
Patients and Lesions
This retrospective review of images and medical records was approved by the our Institutional Review Board. Between March 2004 and October 2006, a search of medical records revealed 145 consecutive patients presenting with abnormal nipple discharge. All patients underwent a mammography and US to evaluate the cause of nipple discharge, which was an initial work-up protocol at our institution. Suspicious abnormalities were found in 95 patients. Among these patients, US-guided vacuum-assisted removal was performed in 50 patients with an intraductal mass revealed by US. Patients with abnormal nipple discharge and an intraductal mass by US that subsequently underwent surgery due to biopsy results of atypia (n = 6) or malignancy (n = 2), and those without follow-up data (n = 4) or a follow-up examination less than 12 months after the treatment (n = 2) were excluded from the study. The 36 remaining patients (age range, 18-80 years; mean age, 46 years) with serous (23 of 36, 64%) or bloody (13 of 36, 36%) nipple discharge constituted the study population.
A mammography was performed in all 36 patients. Twenty (56%) patients had a negative finding, four (11%) had a mass, two (6%) had microcalcifications, one (3%) had a mass with microcalcifications, and nine (25%) had a focal asymmetry. No patient underwent a galactography.
US Evaluation and US-Guided Directional Vacuum-Assisted Removal (US-DVAR)
US examinations were performed by one of eleven radiologists who specialized in breast imaging using a high-resolution sonographic unit equipped with a 10- or 12-MHz linear transducer (Voluson 730, Kretz, Austria; HDI 5000, Advanced Technology Laboratories, Bothell, WA). Patients were placed in the supine or supine-oblique position before undergoing the US. Radial and antiradial US scans were performed by tracing the discharging duct, lactiferous duct, and trigger points to identify and localize the cause of the nipple discharge.
US-DVAR was performed using an 11-gauge vacuum-assisted device (Mammotome; Ethicon-Endosurgery, Cincinnati, OH) under the guidance of a high-resolution sonographic unit with the 10- or 12-MHz linear transducers mentioned above. The patient's skin was sterilized and about 10-15 mL of 1% lidocaine hydrochloride was injected into the skin, subcutaneous fat, and tissues around the dilated duct. A 4-mm skin incision was then made with a scalpel. Next, a probe was inserted either through or directly subjacent to the lesion and then manually rotated to sample both the target lesion and adjacent tissues in a clockwise manner. We attempted to remove all evidence of target lesions under continuous US monitoring. The mean number of core samples obtained was 9.3 (range: 4-22). Pre- and post-biopsy US images were obtained for all lesions. The entire procedure took approximately 20-30 minutes. Fourteen (39%) procedures were performed by one of three attending radiologists with 2-8 years of breast imaging and intervention experience, 22 (61%) procedures were performed by one of eight fellows who conducted their first 5 to 10 biopsies under the supervision of an attending radiologist.
Postprocedural Management Protocol
Pathology results were compared with relevant imaging findings to determine concordances and discordances of the biopsy results. Patients with a biopsy finding indicating a malignancy or of a high-risk lesion underwent a surgical excision. If the lesion had a highly suspicious sonographic finding, a surgical excision was recommended, despite a benign pathologic result. If the imaging findings were determined to be concordant with a benign pathology, six, 12 and 24 month follow-ups were recommended.
The presence of nipple discharge or a residual lesion, such as solid nodules, were evaluated by follow-up US and clinical records.
Data Collection and Analysis
Radiologists who performed the procedures recorded the lesion size (maximal diameter on US), lesion location, final assessment category according to the Breast Imaging Reporting and Data System (BI-RADS) (
12), and the number of core samples taken during the procedure. The presence of abnormal nipple discharge as well as visible residual lesion were recorded on US examination reports during follow-up US.
Symptom elimination was defined as the absence of nipple discharge on the latest US examination report. We evaluated whether symptom elimination was affected by lesion variables (lesion size, location including distance from the nipple, and BI-RADS category), biopsy variables (number of core samples, radiologist experience), the presence of a residual lesion by follow-up US, and histologic findings. The χ2 test, Fisher's exact test, and the Student's t-test were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL), and results were deemed statistically significant at a two-sided significance level of 5%.
Go to :

RESULTS
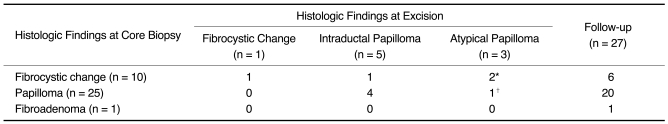
Of the 36 lesions, 69% (25 of 36) were intraductal papillomas, 28% (10 of 36) were attributed to fibrocystic change, whereas 3% (1 of 36) was a fibroadenoma based on the US-DVAR histology. Subsequent surgical excision was performed in nine patients (mean 8.1 ± 7.2 months after US-DVAR, range 1-21 months) due to persistent or recurrent nipple discharge (n = 7, 78%), radiologic-pathologic discordance (n = 1, 11%) without nipple discharge, or an increase in the size of another solid nodule (n = 1, 11%). The target surgical excision sites were assessed by follow-up US. Skin marking for the target lesion was performed in eight patients.
The remaining 27 patients underwent a follow-up US. No malignancy was found in either the nine patients who underwent surgical excision or the 27 who underwent a follow-up US (
Table 1).
Table 1
Comparison of US-Guided Directional Vacuum-Assisted Removal Results with Surgical Excision

US-DVAR eliminated the nipple discharge in 69% (25 of 36) of the patients (
Fig. 1), the other 31% (11 of 36) remained symptomatic (
Fig. 2). The mean follow-up duration was 25 months (range, 12-42). One (4%, 1 of 27) patient underwent a final follow-up at 12 months, nine patients (33%, 9 of 27) at 13-24 months, and 17 patients (63%, 17 of 27) at 25-42 months. At the initial visits after US-DVAR, nipple discharge had disappeared in 81% (29 of 36) of patients. However, four of these 29 later experienced symptom recurrence (2 at 6 to 12 months and 2 at 19 months; 3 intraductal papillomas and 1 fibrocystic change, by surgical excision).
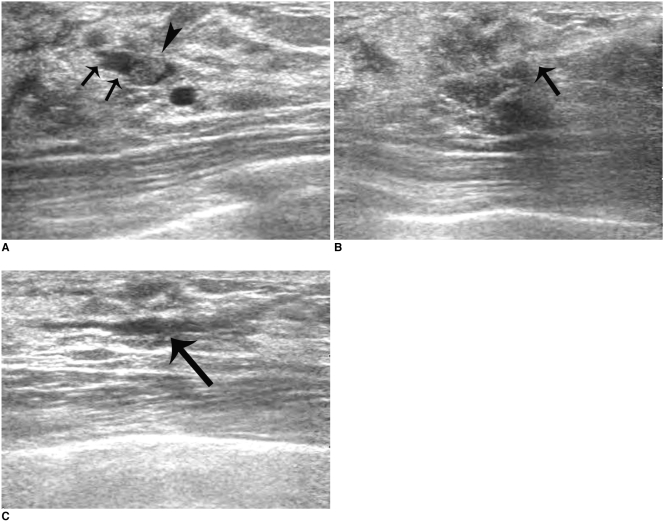 | Fig. 1
44-year-old woman successfully treated for bloody nipple discharge by US-guided directional vacuum-assisted removal.
A. Sonogram showing well-defined, intraductal single mass (arrowhead) within dilated duct (arrows).
B. Sonogram showing directional vacuum-assisted probe (arrow) underneath lesion.
C. Sonogram obtained immediately after US-guided directional vacuum-assisted removal revealing mild fluid collection within dilated duct (arrow). Mass was confirmed to be intraductal papilloma. No residual nipple discharge was apparent at six month follow-up.

|
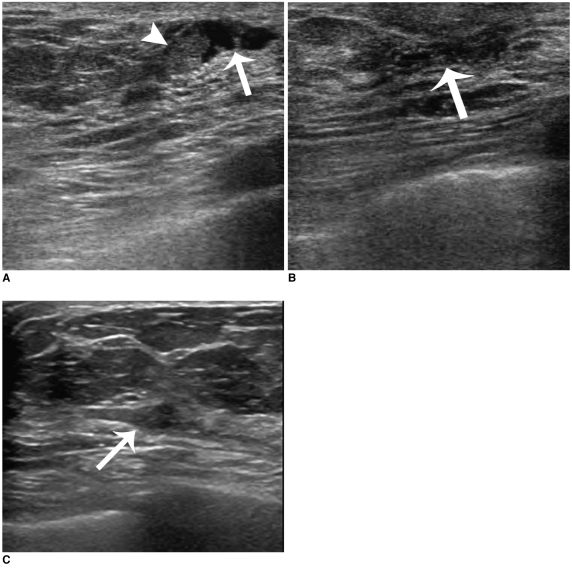 | Fig. 2
48-year-old woman with remaining bloody nipple discharge symptom after US-guided directional vacuum-assisted removal.
A. Sonogram showing well-defined, single intraductal mass (arrowhead) within dilated duct (arrow).
B. Sonogram obtained immediately after US-guided directional vacuum-assisted removal revealing mild fluid collection within slightly dilated duct (arrow). Mass was confirmed as intraductal papilloma.
C. Follow-up sonogram six months after US-guided directional vacuum-assisted removal showing low echoic mass (arrow) in subareolar area. Nipple discharge persisted and biopsy revealed recurrent intraductal papilloma.

|
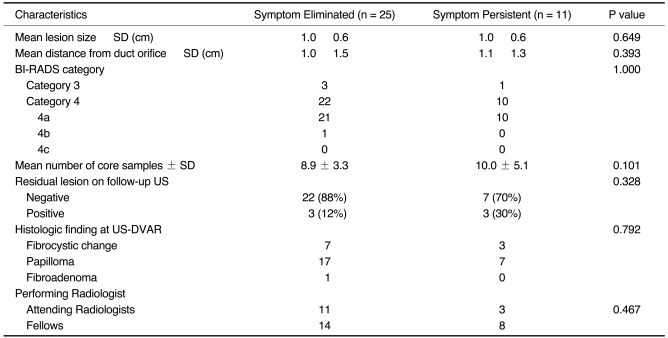
The characteristics of groups with eliminated and persistent nipple discharge are summarized in
Table 2. Mean lesion sizes were 1.0 cm and 1.0 cm (
p = 0.649), whereas the mean lesion distances from the nipple were 1.0 cm and 1.1 cm, respectively (
p = 0.393). No difference was observed between these two groups in terms of BI-RADS category (
p = 1.000) the number of core samples (
p = 0.101), radiologist experience (i.e., attending radiologists versus fellows) (
p = 0.467), the presence of a residual lesion on follow-up US (
p = 0.328), and histologic results by US-DVAR (
p = 0.792). Of the four lesions with fibrocystic change that underwent surgical excision, one benign papilloma, two atypical papillomas, and one fibrocystic change were revealed (
Table 1). Of the six lesions that showed a fibrocystic change and the one fibroadenoma, which was not excised, no case showed a persisting symptom or symptom recurrence over a mean follow-up of 31 months (range 23-42).
Table 2
Characteristics of Groups with Eliminated or Persistent Nipple Discharge Symptoms Following US-Guided Directional Vacuum-Assisted Removal of Benign Intraductal Single Mass

Go to :

DISCUSSION
Of the 36 patients with abnormal nipple discharge, US-DVAR eliminated nipple discharge in 25 (69%) at a mean follow up of 25 months. At the six month follow-ups, nipple discharge was absent in 29 of the 36 (81%). However, four of the 36 (11%) developed the symptom later, and three of these four patients were found to have intraductal papilloma at surgery. Considering the 95-97% resolution rate reported in previous studies (
10,
11), the rate found in the present study is poorer than expected. There are some possible causes for the lower outcome of this study. First, the key to eliminating nipple discharge is to correctly identify and remove the cause, and in the present study, target lesions within dilated ducts were traced to discharging nipple ducts by radial and antiradial US alone. However, as described by Rissanen et al. (
13), a certain number of lesions invisible to US, are visualized with a galactography. Furthermore, Dennis et al. (
10) evaluated symptom causes by repeated ductographic US correlation, and found that this improved correct target lesion identification. In addition, US has been shown to be limited in terms of the detection of small intraductal lesions in peripheral ducts, especially when they are located at 3 cm or more from the nipple (
13,
14). Second, although we attempted to remove all evidence of intraductal lesions by US-DVAR in patients that presented with discharge, there was a substantial probability of residual lesion not visible during procedure (
8), which might cause recurring symptoms later. In our study, a follow-up US revealed residual lesions near the DVAR site in 30% of patients with recurrent or persistent symptoms. Furthermore, US-DVAR was performed in the present study by one of eight fellows or three attending radiologists, and symptom recurrence was slightly lower in the group performed by the attending radiologists compared to the fellows (3 of 14 [21%] versus 8 of 22 [36%];
p = 0.467), although the difference was not statistically significant. Variability of their experience could have affected the lower resolution rate of US-DVAR.
In the present study, we also evaluated the factors affecting symptom resolution to identify a subset of patients that could be applied to the US-DVAR. However, lesion size, location, BI-RADS category, number of core samples, and the presence of a residual lesion by follow-up US were found to be unrelated to symptom resolution.
In terms of histologic findings, 69% (25 of 36) of the lesions were papillomas, and the other 31% were fibrocystic change (n = 10) or fibroadenomas (n = 1). Of the four cases of fibrocystic change with the remaining symptom, surgical excision revealed one benign papilloma and two atypical papillomas where the cause of discharge might have not been accurately targeted during US-DVAR. However, earlier researches reported that 20% of nipple discharge cases were caused by fibrocystic changes or ductectasia (
11,
13), and often appeared as intraductal echogenic lesions on US. In the present study, six cases with fibrocystic changes and one case with fibroadenomas in whom symptoms did not recur at the mean follow-up period of 31 months (range 23-42), were considered to have been accurately targeted during US-DVAR.
In terms of the diagnostic accuracy of US-DVAR, no malignant lesion was found among the nine lesions treated surgically or among the 27 lesions followed-up. The five lesions with papilloma at biopsy histology were found to be benign papillomas in four cases and atypical papilloma in one case at surgery. Recent studies have concluded that benign papillomas detected by core needle biopsy should be surgically excised, because 21-31% of the benign papillary lesions were upgraded to high-risk lesions or cancer at surgery (
15,
16). However, it remains to be determined whether an US-DVAR diagnosis of benign papilloma can obviate the need for surgical excision, which is beyond the scope of this study.
The present study has some limitations. First, we did not perform a consecutive case collection and include all symptomatic patients. Second, the sample size is small. Third, follow-up period is not long enough to evaluate recurring symptoms or establish the benignity. We included only patients with more than 12-month follow-up in the study. However, to confirm the benignity of the lesion, a 24-month follow-up is required. Fourth, we did not perform galactography to identify the cause of abnormal nipple discharge. Small peripheral intraductal lesions, which are not visible on US, may cause residual symptoms. To establish the management of nipple discharge with intraductal solitary lesions on US, the proper selection of pure intraductal lesions based on galactography and prospective large group study results comparing surgical excision and vacuum-assisted removal of intraductal lesions would be needed.
To conclude, US-guided directional vacuum-assisted removal of a benign intraductal mass eliminated nipple discharge in only 69% of patients and thus, this modality should not be considered as an alternative to surgical excision.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download