Abstract
Materials and Methods
Twenty-nine narcoleptic patient with cataplexy and 29 age and sex-matched normal subjects (mean age, 31 years old) underwent volumetric MRIs. The MRIs were spatially normalized to a standard T1 template and subdivided into gray matter, white matter, and cerebrospinal fluid (CSF). These segmented images were then smoothed using a 12-mm full width at half maximum (FWHM) isotropic Gaussian kernel. An optimized voxel-based morphometry protocol was used to analyze brain tissue concentrations using SPM2 (statistical parametric mapping). A one-way analysis of variance was applied to the concentration analysis of gray matter images.
Results
Narcoleptics with cataplexy showed reduced gray matter concentration in bilateral thalami, left gyrus rectus, bilateral frontopolar gyri, bilateral short insular gyri, bilateral superior frontal gyri, and right superior temporal and left inferior temporal gyri compared to normal subjects (uncorrected p < 0.001). Furthermore, small volume correction revealed gray matter concentration reduction in bilateral nuclei accumbens, hypothalami, and thalami (false discovery rate corrected p < 0.05).
Narcolepsy is characterized by excessive daytime sleepiness (EDS), a disruption of sleep-wake behavior, cataplexy (a sudden loss of muscle tone provoked by emotional stimuli), and other rapid eye movement (REM) sleep phenomena, such as, sleep paralysis and hypnagogic hallucinations (1). Numerous investigations, including neuroimaging studies, have been performed to characterize the pathophysiology of narcolepsy. An abnormality of the pontine reticular formation, where REM sleep is generated, was reported in the brain MRIs of three idiopathic narcoleptic patients (2), whereas another study found no evidence of a pontine lesion (3).
Several voxel-based morphometry (VBM) studies of brain MRIs in narcoleptics have produced controversial results. One distinct VBM study revealed reduced gray matter (GM) concentrations in the hypothalamus and nucleus accumbens (4). A subsequent study failed to find any changes of GM concentrations in the hypothalamus (5). Another study found reductions in bilateral inferior temporal and frontal regions (6). Recently, GM loss in the prefrontal and frontomesial cortices was found, but could not provide functional significance with respect to the relevance to the pathophysiology of narcolepsy due to inhomogeneous patient groups and small sample size (7).
In the present study, we enrolled narcoleptics with cataplexy. A VBM analysis was performed on the brain MRIs of narcoleptic patients and normal subjects to identify and compare cerebral structural abnormalities in the narcoleptics with cataplexy.
We consecutively recruited 32 patients with narcolepsy that visited the university hospital sleep center. The recruitment criteria included patients with no central nervous system (CNS) stimulant or cataplexy drug treatment history. The diagnosis of narcoleptics with cataplexy was made according to the revised International Classification of Sleep Disorders. The presence of cataplexy was determined by criteria outlined by Mignot et al. (8) and include: 1) loss of muscle tone has a visible effect or involves other muscle groups in addition to leg muscles; 2) frequency of cataplexy occurring > 1 per month; 3) duration of cataplexy (often or always) < 10 min; 4) cataplexy (often or always) associated with normal state of consciousness.
A standard polysomnographic study comprising one overnight recording followed by a MSLT (multiple sleep latency test) was also performed. MSLT consisted of five naps scheduled at 2-hour intervals starting at 9:00 AM. Patients were invited to lie down on a bed in a dark, sound-attenuated room and instructed to try to fall asleep. Sleep latency was defined as the time elapsed from the start of the test (lights out) to the first 30-second epoch scored as sleep. Each sleep latency test was ended 20 minute after the onset of sleep or after 20 minute of wakefulness. A sleep onset REM period (SOREMP) was defined as one or more epochs of REM sleep occurring within 15 minute of the first 30-second epoch scored as sleep.
Subjects with a mean sleep latency of ≤ 8 minute on the MSLT were evaluated for HLA-DQB1*0602 and DRB1*1501. Other information including the presence of sleep attacks, hypnagogic hallucinations, sleep paralysis, and a positive family history of narcolepsy was obtained from patients and their families. Three of the patients were excluded because they had concomitant mild to moderate obstructive sleep apnea hypopnea syndrome. Finally, a total of 29 narcoleptics with cataplexy were included.
Twenty-nine normal subjects that responded to a local community advertisement and fulfilled a detailed clinical interview, sleep questionnaire, overnight polysomnography, which were evaluated and interpreted by two sleep medicine specialists. Exclusion criteria included a normal subject who showed an apnea-hypopnea index (AHI) > 4 or evidence for other sleep disorders such as periodic limb movement disorders on polysomnography.
The exclusion criteria for normal subjects were those with a 1) mean daily sleep time < 7 hours, 2) abnormal sleep-wake rhythm, 3) other sleep disorders, 4) heart or respiratory disease, 5) history of cerebrovascular disease, 6) other neurological (neurodegenerative diseases, epilepsy, head injury) or psychiatric diseases (psychosis, current depression), 7) alcohol or illicit drug abuse or current intake of psychoactive medications, and 8) a structural lesion on brain MRI.
All patients and normal subjects granted written informed consent before an MRI scan was performed and the Institutional Review Board at Samsung Medical Center authorized the informed consent form and study protocol, which included an MRI scan.
The human leukocyte antigen (HLA) plays a key role in autoimmune disease etiology. As one component of the trimolecular complex (major histocompatibility complex-peptide-T-cell receptor), the presence of specific HLA alleles determines the repertoire of peptide epitopes that can be presented, thereby restricting the specificity of reactive T cells (9). The HLA class II region genes DQB1*0602 and DRB1*1501 are currently the best genetic predictors for narcolepsy in humans (9, 10).
Sequence-specific primers and a BigDye Terminator v. 3.1 cycle Sequencing Kit (Applied Biosystems, Foster City, CA) were used for HLA-DQB1*0602 and DRB1*1501 genotyping according to manufacturer's instructions (Applied Biosystems).
MRI scanning was performed using a GE Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI). T1-weighted spoiled gradient recalled (SPGR) coronal images were obtained using the following scanning variables; 1.6 mm thickness, no gap, 124 slices, repetition time/echo time (TR/TE) = 30/7 msec, flip angle (FA) = 450, number of excitations (NEX) = 1, matrix = 256 × 192, and field of view (FOV) = 22 × 22 cm. The investigators performing the MRI were blinded as to the order of the subject status was (patients versus normal subjects).
Using SPM2 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London, UK) and MATLAB 6.5 (The MathWorks, MA), an optimized VBM protocol was used to analyze brain tissue concentrations. The brain center point was placed on the anterior commissure.
To create customized templates and prior images of GM, all MRIs of narcoleptic patients and normal controls were spatially normalized against the standard MNI (Montreal Neurological Institute) T1 SPM template. Spatial normalizations were applied using the following a voxel size of 1×1×1 mm, cutoff spatial normalization, a 25 mm cutoff' nonlinear regularization, medium regularization, and 16 nonlinear iterations. The normalized images were subdivided into GM, white matter (WM), and CSF spaces, and then sub-sampled for voxel sizes of 2×2 ×2 mm. To remove the isolated voxels of one tissue class which were unlikely to be a member of this tissue type, the Hidden Markov Random Field model was applied in all segmentation processes. Spatially normalized raw images, as well as GM and WM were averaged and saved into customized T1 templates, GM, WM, and CSF prior images, respectively. Finally, the customized T1 template, GM and WM prior images were smoothed using an 8-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel (IGK).
The raw T1 images of all subjects (n = 102) were automatically subdivided into GM, WM, and CSF partitions in native space. Spatial normalization parameters were estimated by matching GM with an own GM template, and then spatially normalized versions of the original images were created. The spatially normalized images were segmented using our own images taken beforehand (GM, WM, and CSF partitions). The GM images were smoothed using a 12-mm FWHM IGK and the final voxel size was 1×1×1 mm.
An ANCOVA covariate with age was used for the concentration analysis of GM images. The significance level (height threshold) was set to an uncorrected p value of < 0.001. In addition, cluster sizes less than 200 voxels were excluded (extent threshold, kE < 200 voxels). Partial correlation analyses were set up with confounding factors such as age between gray matter concentrations (GMCs) and age of EDS onset, disease duration, mean sleep latency of MSLT, or Epworth sleepiness scale were performed using SPM2 using a whole brain mask.
The mean values of the significant clusters were extracted from GM images at an uncorrected p-value < 0.001.
Age, EDS or cataplexy onset age, mean ESS (Epworth sleepiness scale) and SSS (Stanford sleepiness scale), and night polysomnographic findings were analyzed with the two-tailed t-test.
All patients examined were right-handed. The mean age of the patients and normal subjects (M:F = 15:14) was 31.2 years. The onset age of EDS in patients was 22.3 years (range of age: 8-37) and the cataplexy was 23.1 years (9-41). Twenty-three patients (79%) have suffered from hypnagogic or hypnapompic hallucinations and 19 patients (65%) had a history of sleep paralysis. The mean Epworth sleepiness scale result was 17.5 in patients versus 4.3 in normal subjects (t-test, p < 0.01). Positive HLA typing (DR2 and DQB1*0602) was identified in 27 (93%) of the studied patients. The sleep study findings in patients and normal subjects were summarized in Table 1 (normal subjects underwent only night polysomnography).
All patients and normal subjects underwent a brain MRI using the same protocol, which revealed no gross abnormal findings on visual inspection.
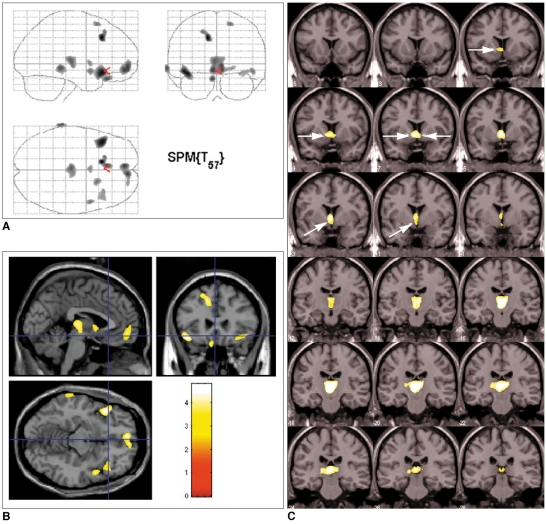
Compared to normal subjects, narcoleptics with cataplexy showed reduced GM concentrations in bilateral thalami, left gyrus rectus, bilateral frontopolar gyri, bilateral superior frontal gyri, bilateral anterior short insular gyri, right superior temporal gyrus, and left inferior temporal gyrus at the level of uncorrected p < 0.001 (Fig. 1A, B) (Table 2). There were no regions of the brain that showed increased GM concentrations in narcoleptics with cataplexy. Using small volume corrected analysis, the GM concentration was significantly reduced in bilateral nuclei accumbens, bilateral hypothalami, and at bilateral thalami at the level of a false discovery rate p < 0.05 (Fig. 1C).
A partial correlation analyses with the confounder of age between GMCs and age of EDS onset, disease duration, mean sleep latency of MSLT, or Epworth sleepiness scale did not show statistically significant results.
In the present study, a VBM analysis was performed on the brain MRIs to identify cerebral structural abnormalities in the narcoleptics with cataplexy. VBM methods for GM concentrations refer to differences observed in the proportion of GM voxels, which are defined based on signal intensity thresholds, compared to voxels representing other tissue types as GM density differences (13). On the contrary, the thickness of the cerebral cortex (ranging between 1.5 to 4.5 mm) reflects the density and arrangement of cells (neurons and neuroglia and nerve fibers) (14). Our study demonstrates a significant decrease in GM concentrations in the hypothalamus, nucleus accumbens, thalamus, anterior insular cortex, and in some cortical areas in the frontal and temporal lobes of the brains of patients with narcolepsy with cataplexy, when compared to normal subjects.
In the present study, reduced GM concentrations in the bilateral hypothalami and nuclei accumbens may be related to a decreased number of hypocretin immunoreactive neurons in the hypothalamus of a narcoleptic brain (15). The neuropeptide hypocretin exists exclusively in the posterior hypothalamus of the human brain and plays a critical role in the neurobiology of narcolepsy (15). Our previous FDG-PET (fluorodeoxyglucose-positron emission tomography) (16) and SPECT (single photon emission computed tomography) studies (17) showed significant glucose hypometabolism and hypoperfusion in the bilateral hypothalami of narcoleptics. A recent proton MR spectroscopy study revealed that the hypothalamic N-acetylaspartate-to-creatine ratio was significantly lower in narcoleptics with cataplexy, when compared to narcoleptics without cataplexy or in normal subjects (18). The nucleus accumbens has been implicated in involvement in the regulation of the sleep-wake cycle via the mesolimbic-dopamine system (19), and in the interface between the limbic and motor systems (20, 21). However, others were unable to find similar changes in the hypothalamus or nucleus accumbens (5-7). One study suggested that the absence of detectable structural changes in hypothalamus and in hypocretin projection areas may be due to microscopic changes in these areas that are not detected by VBM, or alternatively, that functional abnormalities of hypocretin neurons are not associated with structural correlates (5). Other VBM studies found that a bilateral reduction of GM concentrations in inferior temporal and frontal brain regions (22) or in prefrontal and frontomesial regions (7). They acknowledged that the functional significance of these findings was unclear, but suggested that the involvement of a presumed autoimmune mediated process that destroys hypothalamic hypocretin neurons and extends to other neuronal populations. As pointed out, inhomogeneous patient groups, a stimulant or antidepressant medication history, and small sample sizes may have caused the negative findings of previous VBM studies (7). Because narcolepsy is composed of different subgroups, i.e., narcoleptics with or without cataplexy, and narcolepsy with a reduced or a normal hypocretin level in CSF, it is important that homogenous subgroups of narcoleptic patients are identified in studies to facilitate accurate intergroup comparisons. The findings of the present VBM study, which included reduced GM concentrations in the hypothalamus and nucleus accumbens, may strengthen the prior hypothesis that these reductions are associated with EDS and cataplexy in narcolepsy cases.
Intralaminar nuclei are included in the 'nonspecific ascending reticular activating system' and are functionally associated with the sense of attention, arousal, and consciousness (22). These central nuclei are also involved in the motor function in the basal ganglia circuitry and in cognitive, oculomotor, and limbic functions (23). The anterior thalamic nucleus belongs to the Papez circuit, the neural circuit of emotion. The median thalamic nucleus has been implicated in the wake-sleep cycle (24). A decrease in metabolism and regional cerebral blood flow in the thalamus of the narcoleptic brain, have been demonstrated by previous neuroimaging studies (16, 17). Thus, the reduction of GM concentrations in bilateral thalamic nuclei may be related to attention or cognition deficits, memory impairments as well as arousal and sleep-wake disturbances in narcolepsy.
Depressive and neurotic symptoms are considered to be common in narcolepsy (25). Some have suggested that significant hypoperfusion of the limbic system may be related to the emotional instability seen in narcoleptics (17). The insular cortex has numerous connections with the cerebral cortex, basal ganglia, and limbic structures (26, 27). Mesial temporal lobe epilepsy patients with emotional symptoms have shown hypometabolism in the anterior insula by FDG-PET (28, 29). A GMC decrease in the anterior insular cortex found in narcoleptics with cataplexy suggested that a structural abnormality in that region may be associated with the cataplexy induced by emotional changes.
We applied two types of statistical analyses, namely, an unpaired t-test for the group comparisons between the narcoleptic patients and normal subjects; and a small volume correction (SVC) on the prior hypothesis, which mentions the hypothalamus, nucleus accumbens, and thalamic dysfunction in narcoleptics. The results obtained by the two methods were similar for the hypothalamus, nucleus accumbens, and thalamus. The use of SVC in a SPM analysis is a widely accepted method. A SVC can be applied when there is prior knowledge or consensus on an activation effect of a particular region instead of the entire brain (12, 30). Any clinical or animal experimental data can be the supportive evidence for a SVC analysis. SPM99 and later versions, use the results to calculate corrected statistics across the whole brain by working out the shape and size of the whole brain volume in the analysis, and calculating the correction accordingly (11). Thus, for a whole-brain analyses, SPM99 can offer a reasonable correction factor for the entire brain volume. For smaller volumes, the correction must take into account the geometric properties of the volume, such as shape and surface area. This is important, because you may well have an a priori hypothesis to test an area of expected activation in a SPM. Because multiple comparisons across the whole brain image are too conservative, one must restrict their region of interest to a subset area instead of using the whole brain image. Worsley et al. (11) gives results which allow for the choice of appropriate thresholds given that you are restricting your investigation to a certain volume of interest, defined shape, size, and so on. This is because both size and shape dictate how many resels the volume will contain. As the random fields tutorial explains, the number of resels in a volume is a measure related to the number of independent observations in that volume, and this in turn will dictate how strict our correction must be (http://imaging.mrc-cbu.cam.ac.uk/imaging/SmallVolumeCorrection).
In conclusion, this study revealed significant GM concentration deficits in narcoleptics with cataplexy. Those areas included structures that may serve possible roles in wake-sleep controls, attention, or memory. These findings would be helpful in elucidating the pathomechanism of cerebral disturbances in patients with narcolepsy with cataplexy.
Notes
This study was supported by a Grant (2009K001257) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science, by a grant (no. A090579) of Good Health R&D Project, Ministry of the Health & Welfare, Republic of Korea, and by the Samsung Biomedical Research Institute grant, #SBRI C-A7-226-3, and by the Samsung Medical Center Clinical Research Development Program Grant, #CRS-108-11-1.
References
1. Guilleminault C, Dement WC. 235 cases of excessive daytime sleepiness. Diagnosis and tentative classification. J Neurol Sci. 1977; 31:13–27. PMID: 188992.
2. Plazzi G, Montagna P, Provini F, Bizzi A, Cohen M, Lugaresi E. Pontine lesions in idiopathic narcolepsy. Neurology. 1996; 46:1250–1254. PMID: 8628461.

3. Bassetti C, Aldrich MS, Quint DJ. MRI findings in narcolepsy. Sleep. 1997; 20:630–631. PMID: 9351130.

4. Draganski B, Geisler P, Hajak G, Schuierer G, Bogdahn U, Winkler J, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002; 8:1186–1188. PMID: 12411926.

5. Overeem S, Steens SC, Good CD, Ferrari MD, Mignot E, Frackowiak RS, et al. Voxel-based morphometry in hypocretin-deficient narcolepsy. Sleep. 2003; 26:44–46. PMID: 12627731.
6. Kaufmann C, Schuld A, Pollmächer T, Auer DP. Reduced cortical gray matter in narcolepsy: preliminary findings with voxel-based morphometry. Neurology. 2002; 58:1852–1855. PMID: 12084891.

7. Brenneis C, Brandauer E, Frauscher B, Schocke M, Trieb T, Poewe W, et al. Voxel-based morphometry in narcolepsy. Sleep Med. 2005; 6:531–536. PMID: 15994127.

8. Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997; 20:1012–1020. PMID: 9456467.
9. Doherty DG, Penzotti JE, Koelle DM, Kwok WW, Lybrand TP, Masewicz S, et al. Structural basis of specificity and degeneracy of T cell recognition: pluriallelic restriction of T cell responses to a peptide antigen involves both specific and promiscuous interactions between the T cell receptor, peptide, and HLA-DR. J Immunol. 1998; 161:3527–3535. PMID: 9759873.
10. Chabas D, Taheri S, Renier C, Mignot E. The genetics of narcolepsy. Annu Rev Genomics Hum Genet. 2003; 4:459–483. PMID: 14527309.

11. Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. An unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapping. 1996; 4:58–73.
12. Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000; 27:469–474. PMID: 11055430.

13. Ashburner J, Friston KJ. Voxel-based morphometry -- the methods. Neuroimage. 2000; 11:805–821. PMID: 10860804.
14. Parent A, Carpenter MB. Human neuroanatomy. 1995. Baltimore, MD: Williams & Wilkins.
15. Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000; 20:7760–7765. PMID: 11027239.

16. Joo EY, Tae WS, Kim JH, Kim BT, Hong SB. Glucose hypometabolism of hypothalamus and thalamus in narcolepsy. Ann Neurol. 2004; 56:437–440. PMID: 15349874.

17. Joo EY, Hong SB, Tae WS, Kim JH, Han SJ, Cho YW, et al. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005; 28:410–416. PMID: 16098766.

18. Lodi R, Tonon C, Vignatelli L, Iotti S, Montagna P, Barbiroli B, et al. In vivo evidence of neuronal loss in the hypothalamus of narcoleptic patients. Neurology. 2004; 63:1513–1515. PMID: 15505179.

19. Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000; 126:3–28. PMID: 11105636.

20. Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003; 160:1726–1739. PMID: 14514480.

21. American Academy of Sleep Medicine. The International Classification of Sleep Disorders. Diagnostic & Coding Manual. 2005. 2nd ed. Westchester: The American Academy of Sleep Medicine.
22. Percheron G, François C, Talbi B, Meder JF, Fenelon G, Yelnik J. The primate motor thalamus analysed with reference to subcortical afferent territories. Stereotact Funct Neurosurg. 1993; 60:32–41. PMID: 8511432.

23. Sadikot AF, Parent A, François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992; 315:137–159. PMID: 1372010.

24. Akopyan NS, Baklavadzhyan OG, Sarkisyan NV. The effects of the mediodorsal nucleus of the thalamus on respiratory neurons of the medulla oblongata and respiration in rats in conditions of hypoxia. Neurosci Behav Physiol. 2000; 30:449–453. PMID: 10981949.

25. Lishman A. Organic Psychiatry. 1998. 3rd ed. Oxford: Blackwell.
26. Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997; 17:9686–9705. PMID: 9391023.

27. Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987; 262:271–289. PMID: 3624555.

28. Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol. 2002; 51:202–208. PMID: 11835376.

29. Wehner T, Luders H. Role of neuroimaging in the presurgical evaluation of epilepsy. J Clin Neurol. 2008; 1:1–16. PMID: 19513318.

30. Lee JD, Park HJ, Park ES, Kim DG, Rha DW, Kim EY, et al. Assessment of regional GABA(A) receptor binding using 18F-fluoroflumazenil positron emission tomography in spastic type cerebral palsy. Neuroimage. 2007; 34:19–25. PMID: 17049274.

Fig. 1
Brain regions showing reduced gray matter concentrations in patients with narcolepsy and cataplexy.
A. Overall areas showing reduced gray matter concentrations as whole are shown in glass brain view.
B. Decreased gray matter concentrations in narcoleptics with cataplexy in: left gyrus rectus, bilateral thalami, bilateral frontopolar gyri, bilateral short insula gyri, bilateral superior frontal gyri, right superior temporal gyrus, and left inferior temporal gyrus are shown as T1 template overlaid MR image. Uncorrected p < 0.001 (extent threshold kE < 100 voxels).
C. Bilateral nuclei accumbens (dotted arrows), bilateral hypothalamus (solid arrows), and bilateral thalami (arrowhead) showed reduced gray matter concentrations at false discovery rate level of corrected p < 0.05 with small volume correction. Superior to inferior panels are arranged in anterior to posterior direction in coronal images. Left-hand sides of images represent left side of brain.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download