Abstract
Over the last few years, percutaneous radiofrequency (RF) ablation has been successfully established as a viable treatment modality for small peripheral renal cell carcinoma (RCC). This technique is limited by central tumor location and tumor size. We report the interventional management of a 5.3 cm mixed RCC with central and exophytic parts by combining the RF ablation with embolization, tagging, and retrograde, as well as anterograde cooling. The potential pitfalls of complex hybrid interventions for treating RCC are discussed.
Renal cell carcinoma (RCC) is the most frequent malignancy of the kidneys. The increasing availability of US, CT and MR imaging in recent years has led to a higher rate of incidentally diagnosed RCCs. For 2008, more than 54,000 new cases of renal and uropelvic carcinomas are estimated to be detected in the United States alone. The standard therapy for non-metastatic and local RCC is a nephrectomy or nephron-sparing surgery; whereas target drug therapy or immunotherapy is used in metastatic renal cell carcinoma. Minimally invasive options include laparoscopic nephron-sparing surgery, cryoablation, and radiofrequency (RF) ablation. As RCCs typically stay asymptomatic for a long while, the late onset in life implies an increased rate and gravity of comorbidities amongst RCC patients, ultimately leading to a considerably higher demand for the nonsurgical treatment of RCC.
Radiofrequency ablation has become an important therapeutic alternative for different kinds of neoplasms; not only kidney cancer. Percutaneous RF ablation is associated with a relatively low number of undesired side effects and complications due to its minimally invasive and locally acting nature. It is especially useful for patients that are ineligible for or refusing surgery. Various studies reported on the successful application of RF ablation in renal tumors (1-3). One of the most relevant problems is the possible damage to the collecting system during the procedure, which can cause considerable morbidity (2). Moreover, in central or mixed lesions with central and exophytic parts, complete ablation is not consistently achieved with incomplete ablation in up to 39% of patients (4). Therefore, RF ablation is commonly rejected in central renal lesions. Retrograde cooling of the collecting system has been cited as having the ability to overcome this limitation (5, 6). Anterograde chilling of the collecting system via a percutaneous nephrostomy has only been reported in the treatment of transitional cell carcinoma of the renal pelvis (7). Another issue in renal RF ablation is the fact that most renal neoplasms are not visible on non-enhanced CT scans. Therefore the application of a contrast agent is necessary. However, the administration of a potentially nephrotoxic contrast agent may be contraindicated, in particular for patients suffering from renal insufficiency.
We present a case where the combination of multiple interventional techniques led to the solution of a set of treatment-complicating problems, and ultimately to the successful ablation of a central RCC in a patient suffering from renal insufficiency.
A 77-year-old woman presenting with a one month history of progredient dyspnea underwent an unenhanced computed tomography (CT) scan of the chest, which incidentally revealed a largely hyperdense upper pole lesion in the left kidney with a maximum diameter of 5.3 cm (Fig. 1A). Parts of the lesion attained the renal pelvis. This finding was confirmed by ultrasound. An ultrasound-guided fine needle aspiration biopsy revealed RCC of the clear cell type (T1b N0 M0) (Fig. 1). She also suffered from primary hypertension (BP [blood pressure] 140-150/100 mmHg), chronic obstructive pulmonary disease, dilated cardiomyopathy and implantation of a cardiac pacemaker, mitral insufficiency (NYHA [New York Heart Association] III), insulin-dependent diabetes mellitus (fasting glucose value: 100-110 mg/dl), chronic renal failure (creatinine level: 1.5 mg/dl), and alcoholic liver cirrhosis. Due to the markedly elevated perioperative risk (ASA [American Society of Anesthesiology] IV) the patient refused surgery.
Subsequently, the patient was referred for interventional treatment of the tumor. A percutaneous RF ablation was considered to be the most suitable therapeutic option. Due to the size of the lesion, the patient was scheduled for pre-interventional embolization of the central part of her tumor in order to achieve central necrosis and a more homogeneous heat distribution in the devascularized parts of the tumor. Iodinated oil (Lipiodol Ultra-Fluid®, Guerbet, Roissy, France) was considered for lesion tagging since it provides post-embolization contrast and allows for CT-guided placement of the RF-probe without use of additional contrast agent.
Embolization was performed via the right femoral approach using a 4 Fr introducer sheath. After selective catheterization of the left renal artery using a 4 Fr sidewinder catheter (Merit Medical, South Jordan, UT), a 3 Fr microcatheter (Progreat, Terumo, Tokyo, Japan) was placed in the tumor feeding vessels in the upper pole of the kidney. The tumor was subsequently embolized using 10 ml of iodinated oil (Lipiodol, Guerbet, Roissy, France) (Fig. 1B, C). Considering the patient's delicate renal condition, the use of iodinated contrast agent was limited to 10 ml (Visipaque 320, GE Healthcare, Princeton, NJ).
Prior to the RF ablation, a single-J catheter was placed in the left ureter with its tip in the collecting system, to accomplish external renal pelvis cooling. Preliminary testing of the single-J catheter only allowed for a drop-by-drop injection, which was insufficient for cooling and protection from thermal damage. Since the patient rejected conscious sedation, the procedure was performed under general anesthesia. Accordingly, the patient was positioned in the right lateral decubitus position; and, following an unenhanced CT (SOMATOM Sensation 16, Siemens, Forchheim, Germany; 16 × 0.75 mm, 120 kV, 165 mAseff), a CT-guided nephrostomy was performed using the trocar technique with a 6.5 Fr drainage (Resolve, Merit Medical, South Jordan, UT) to achieve sufficient cooling via the anterograde approach. Continuous cooling of the upper calices was performed with pre-chilled (8℃) 0.9% saline infused at a flow rate of 500 ml/h.
For RF ablation, four grounding pads were placed on the patient's proximal thighs. The procedure was carried out using a monopolar RF system (RF 3000, Boston Scientific Corporation, Natick, MA) in combination with a 5 cm umbrella-shaped LeVeen probe (Boston Scientific). Prior to the RF probe placement, the distal 1 cm of the probe insulation was removed to allow for post-interventional coagulation of the puncture tract. The electrode was placed under CT-guidance in the centre of the lesion (Fig. 1D, E). After the final probe position was confirmed, energy was applied according to the manufacturer's recommendations for soft tissue ablation. Ablation was performed using the pullback technique at two positions, and achieving two complete roll-offs at each position. The total time of ablation was 74 minutes. Arterial and venous phase CT scan (SOMATOM Sensation 16, Siemens, Forchheim, Germany; 16×0.75 mm, 120 kV, 165 mAseff) with a reduced amount of contrast agent (80 ml Iodixanol, Visipaque, GE Healthcare) showed a completely devascularized tumor, with no sign of any residual contrast enhancement. Thereafter, the RF probe was gradually retracted under continuous coagulation of the puncture tract by applying 20 W.
No early or delayed complications were noted; however, due to her general condition, the patient was temporarily admitted to the intermediate care unit for observation. She recovered well and the single-J splint was removed a day after and the nephrostomy five days after the ablation. The patient was discharged the following day in good condition. A CT performed at eight months after the procedure, showed residual Lipiodol in the area of the ablation without signs of local renal tumor recurrence (Fig. 1F).
The RF ablation of renal tumors has recently been established as a viable treatment modality and is especially well suited in the case of single kidney, hereditary RCC, multiple renal lesions, and patients who are not eligible for surgery or who refuse surgery. Although no randomized trials have been performed, recent data in 100 tumors showed encouraging results with regards to the efficacy and safety of renal RF ablation (1, 4). However, there is no long term outcome data. Small tumor size (< 3 cm) and a peripheral tumor localization are considered to be predictors for successful ablation (1, 2). For larger tumors, additional embolization has been performed to achieve more homogeneous heating, and thereby an improved outcome (2, 10). For centrally located tumors, collecting system cooling has been proposed as a method to protect these structures from thermal damage. Cooling is typically performed in a retrograde way, thereby avoiding another percutaneous approach (5, 6); although, anterograde cooling via nephrostomy is feasible. This, however, has, thus far, only been described for transitional cell carcinoma (7). The unique feature of this case is the combination of different auxiliary techniques that improve the efficacy and safety of the procedure in an otherwise inoperable tumor.
Some authors propose transarterial embolization as the sole treatment for RCC in patients who are unfit for surgery (8). While this technique is known to be effective as a palliative measure in symptomatic patients, there is virtually no data on long term survival after transarterial embolization as sole treatment of RCCs (9). Therefore, we opted for a combined treatment of embolization of the central part of the mixed tumor, followed by the RF ablation as a definitive treatment. The embolization of the tumor has to be considered as a neo-adjuvant approach, to achieve complete ablation in tumors exceeding 3 cm (2, 10). The choice of Lipiodol for this procedure, although not permanently occluding the tumor feeding vessels, allowed for continuous visualization of the critical part of the target without the need for additional contrast agent in a patient with renal insufficiency. In a follow-up scans at eight months after the intervention, there was an iodinated oil residuum visible, indicating ceased perfusion of the treated area. Anterograde cooling via nephrostomy was applied when the initially planned retrograde cooling of the collecting system proved to be insufficient. With the single-J catheter still in place, this allowed for high flow of the chilled saline.
A major disadvantage for the use of multiple auxiliary techniques lies in the fact that the complication rate and the total procedure time increases with each additional measure. Those negative aspects need to be balanced against the benefit the patient can take from these measures. The key advantage of such combined procedures is the feasibility to treat lesions that are otherwise considered unsuited for interventional therapy. Moreover, the synergistic combination of iodinated oil tagging-embolization and internal (retrograde), in addition to an external (anterograde) collecting system, cooling may render the RF-based therapy of complex renal lesions possible, providing a therapeutic option for well selected patients, where no other treatment is feasible.
Figures and Tables
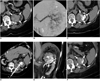 | Fig. 1Renal tumor before (arrows in A), during (* in B), and after (arrows in C) embolization of its central part with iodinated oil. Embolization was focused on central part of mixed tumor in order to achieve sufficient tagging of this area. Multi-planar reformations from intra-procedural CT images (D, E) illustrate positioning of radiofrequency probe and its relation to cooling catheters (* in E, F = RF probe; arrowhead in E = internal catheter; arrows in E = external cooling catheter). Central part of tumor reaches collecting system, which is marked by external cooling catheter. Follow-up CT scan eight months after intervention reveals no signs of local tumor recurrence (F). Note residual iodinated oil at site of ablation (arrows). |
References
1. Gervais DA, Arellano RS, McGovern FJ, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 2, Lessons learned with ablation of 100 tumors. AJR Am J Roentgenol. 2005. 185:72–80.
2. Mahnken AH, Rohde D, Brkovic D, Gunther RW, Tacke JA. Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol. 2005. 46:208–214.
3. Varkarakis IM, Allaf ME, Inagaki T, Bhayani SB, Chan DY, Su LM, et al. Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. J Urol. 2005. 174:456–460.
4. Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005. 185:64–71.
5. Margulis V, Matsumoto ED, Taylor G, Shaffer S, Kabbani W, Cadeddu JA. Retrograde renal cooling during radio frequency ablation to protect from renal collecting system injury. J Urol. 2005. 174:350–352.
6. Rouviere O, Badet L, Murat FJ, Marechal JM, Colombel M, Martin X, et al. Radiofrequency ablation of renal tumors with an expandable multitined electrode: results, complications, and pilot evaluation of cooled pyeloperfusion for collecting system protection. Cardiovasc Intervent Radiol. 2008. 31:595–603.
7. Schultze D, Morris CS, Bhave AD, Worgan BA, Najarian KE. Radiofrequency ablation of renal transitional cell carcinoma with protective cold saline infusion. J Vasc Interv Radiol. 2003. 14:489–492.
8. Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy--natural history, complications, and outcome. Urology. 2004. 64:909–913.
9. Munro NP, Woodhams S, Nawrocki JD, Fletcher MS, Thomas PJ. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003. 92:240–244.
10. Gebauer B, Werk M, Lopez-Hanninen E, Felix R, Althaus P. Radiofrequency ablation in combination with embolization in metachronous recurrent renal cancer in solitary kidney after contralateral tumor nephrectomy. Cardiovasc Intervent Radiol. 2007. 30:644–649.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download