Abstract
Inflammatory pseudotumor, also known as inflammatory myofibroblastic tumor and plasma cell granuloma, is an uncommon low-grade lesion composed of spindle cells admixed with mature plasma cells and other inflammatory cells, such as histiocytes, lymphocytes, and eosinophils. Here, we describe the mammographic and ultrasonographic findings of a case of an inflammatory pseudotumor of the breast in a 60-year-old woman. With the suspicion of malignancy, core needle biopsy and surgical excision confirmed the mass as being an inflammatory pseudotumor of the breast.
Inflammatory pseudotumor of the breast is a rare benign condition. Most inflammatory pseudotumors occur in the lung and airways of young patients. Cases of inflammatory pseudotumors of the breast are scarce, and furthermore, case reports with the imaging findings of that disease entity are even more scarce. Inflammatory pseudotumors of the breast may be solitary or multifocal, have a tendency to enlarge locally, as well as recur after excision (1).
We report a case of an inflammatory pseudotumor of the breast detected during screening mammographic examination.
A 60-year-old woman presented with an abnormality detected by screening mammography. Upon physical examination, we noted a non-tender nodule with a relatively circumscribed margin in the upper outer quadrant of the left breast. The overlying skin showed minimal retraction but no color change. The patient did not have a history of breast injury.
The mammograms revealed a 1.5 cm, ill-defined, high-density mass in the left outer breast (Fig. 1A, B). Within the mass, no associated calcifications were observed.
US showed an irregular shaped, ill-defined homogeneous hypoechoic mass with an echogenic halo (Fig. 1C). Color Doppler study showed moderate vascularity in the peripheral halo portion of the lesion (Fig. 1D). We classified the mass as Breast Imaging Reporting and Data System (BI-RADS) category 4c (moderate suspicion for malignancy - estimated probability for malignancy ranging from 50% to 95%). Next, we performed US-guided automated gun biopsy using a 14-gauge needle and histological examination of the biopsy specimens revealed the presence of an inflammatory pseudotumor. Further, excisional biopsy confirmed this diagnosis.
Upon gross pathology, we did observe an ill-defined pinkish-white mass without necrosis or hemorrhage (Fig. 1E). Microscopically, we noted irregularly oriented intersecting fascicles of spindle cells at low magnification with Hematoxylin and Eosin staining. Mixed inflammatory cells such as lymphocytes, histiocytes, and plasma cells were infiltrated between the spindle cells. At high magnification, the proliferating spindle cells had bland-looking nuclei and the nucleoli were inconspicuous. We did observe mitoses (up to 3 of 10 per high-power field), however, no atypical mitoses were found (Fig. 1F). Following an immunohistochemical assay, we found the spindle cells to be reactive for anti-SMA (smooth muscle actin) and demonstrated myofibroblastic differentiation (Fig. 1G). We also found the tumor cells to be negative for a reaction for pan-CK (pancytokeratin), which resulted in the exclusion of the possibility of metaplastic carcinoma. We also found the tumor cells to be negative for a reaction to anaplastic lymphoma kinase (ALK) (figure not shown).
During a two-year follow up period, we did not find any evidence for tumor recurrence at the left breast, as demonstrated by mammographic and US images.
Inflammatory pseudotumors of the breast are extremely rare; a literature search yielded only fifteen cases in the English-language literature (1-10). In all 15 cases, the original breast inflammatory pseudotumors were unilateral. In addition, we found that all the lesions were surgically excised; however, the three patients showed recurrence after surgery, with two of the three patients having bilateral recurrence (2-4).
The pathogenesis of inflammatory myofibroblastic tumors is controversial; although, the etiology of this entity remains unclear and is considered by some investigators to have an aberrant reactive or inflammatory response to local cytokines in nature (5, 10, 11). However, cases with vascular invasion, local recurrence, and even metastasis have been reported (5, 10-12). Furthermore, the cytogenetic analysis of one case of an inflammatory pseudotumor of the breast showed that the disease was due to clonal proliferation, thus supporting the hypothesis that an inflammatory pseudotumor of the breast is a true neoplasm (7) as opposed to an exuberant tissue response to inflammation. ALK, expressed due to a chromosomal translocation involving 2p23 has recently been demonstrated in some cases of an inflammatory pseudotumor (13, 14).
Radiologically, our case appeared as an ill-defined mass on mammographic and US examination, which is consistent with cases described by Haj et al. (1). However, for most of the previously reported cases, a well-defined border was depicted on mammograms (5). There was also one case with a rare imaging finding, where US demonstrated the presence of a focal area of irregularly marginated acoustic shadowing without a mass configuration (15).
It should be emphasized that the imaging findings of our case were suspicious for malignancy. First, the mass was seen in this case with an ill-defined margin on mammograms and US and the overlying skin was minimally retracted as determined on physical examination, which was also described by Haj et al. (1). Additionally, we depicted the vascularity in the peripheral halo portion of the lesion on color Doppler study. Because the mass was located within a fat layer, fat necrosis could be included in the differential diagnosis.
In conclusion, we have presented a case of an inflammatory pseudotumor of the breast, which was initially misdiagnosed as being malignant due to the ill-defined border of the lesion on mammograms and US. We should consider surgical excision and a close follow-up due to its tendency to enlarge locally and to recur after excision.
Figures and Tables
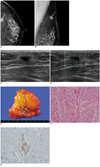 | Fig. 1Inflammatory pseudotumor of breast in 60-year-old woman.
A, B. Left craniocaudal (A) and mediolateral oblique (B) mammograms reveal 1.5-cm-sized ill-defined, high-density mass (arrows) in axillary tail area of left breast.
C. Transverse US scan reveals irregular shaped, ill-defined homogeneous hypoechoic mass with echogenic halo in left axillary tail region. We found nodule surrounded by fat lobules and mass appearing to infiltrate around fat lobules.
D. Color Doppler study reveals moderate vascularity in peripheral halo portion of mass.
E. Upon gross pathology, we observed ill-defined pinkish-white mass (arrowheads) without necrosis or hemorrhage.
F. For microscopic findings at high magnification, proliferating spindle cells had bland-looking nuclei and nucleoli were inconspicuous. There were occasional mitoses (up to 3 of 10 per high-power field), but atypical mitoses were not found (Hematoxylin & Eosin staining, ×200).
G. Following immunohistochemical assay, spindle cells were found to be reactive for anti-SMA (smooth muscle actin), which demonstrates myofibroblastic differentiation (×200).
|
References
1. Haj M, Weiss M, Loberant N, Cohen I. Inflammatory pseudotumor of the breast: case report and literature review. Breast J. 2003. 9:423–425.
2. Yip CH, Wong KT, Samuel D. Bilateral plasma cell granuloma (inflammatory pseudotumour) of the breast. Aust N Z J Surg. 1997. 67:300–302.
3. Zardawi IM, Clark D, Williamsz G. Inflammatory myofibroblastic tumor of the breast. A case report. Acta Cytol. 2003. 47:1077–1081.
4. Khanafshar E, Phillipson J, Schammel DP, Minobe L, Cymerman J, Weidner N. Inflammatory myofibroblastic tumor of the breast. Ann Diagn Pathol. 2005. 9:123–129.
5. Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005. 113:66–69.
6. Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, et al. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005. 29:275–278.
7. Sastre-Garau X, Couturier J, Derre J, Aurias A, Klijanienko J, Lagace R. Inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast. Clinicopathological and genetic analysis of a case with evidence for clonality. J Pathol. 2002. 196:97–102.
8. Chetty R, Govender D. Inflammatory pseudotumor of the breast. Pathology. 1997. 29:270–271.
9. Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988. 90:627–632.
10. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.
11. Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990. 94:538–546.
12. Maier HC, Sommers SC. Recurrent and metastatic pulmonary fibrous histiocytoma/plasma cell granuloma in a child. Cancer. 1987. 60:1073–1076.
13. Cessna MH, Zhou H, Sanger WG, Perkins SL, Tripp S, Pickering D, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002. 15:931–938.
14. Freeman A, Geddes N, Munson P, Joseph J, Ramani P, Sandison A, et al. Anaplastic lymphoma kinase (ALK1) staining and molecular analysis in inflammatory myofibroblastic tumours of the bladder: a preliminary clinicopathological study of nine cases and review of the literature. Mod Pathol. 2004. 17:765–771.
15. Akbulut M, Gunhan-Bilgen I, Zekioglu O, Duygulu G, Oktay A, Ozdemir N. Fine needle aspiration cytology of inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast: a case report and review of the literature. Cytopathology. 2007. 18:384–387.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download