Abstract
We report a case of hypereosinophilia causing multiple areas of cerebral infarcts. A 52-year-old Korean man presented with dysarthria and weakness in both arms. A brain MRI revealed multiple acute infarcts in the distal border zone with focal intracerebral hemorrhage, whereas a cerebral angiogram was not remarkable. The eosinophil count was 5,500/µL and was accompanied by elevated cardiac enzyme levels. The pattern of cerebral infarcts and laboratory results suggest a thromboembolic infarction associated with hypereosinophilia.
Hypereosinophilia is characterized by an elevated eosinophil count (greater than 1,500/µL), and can present with neurological manifestations, including encephalopathy, sensory polyneuropathy and cerebral infarction (1). The cause of cerebral infarction is thought to be a thromboembolism or the cerebrovascular endothelial toxicity of eosinophils (2). A thromboembolism may affect the vascular border zone, which is usually related to infarction from prolonged systemic hypotension or proximal carotid arterial obstruction (3).
We report a patient with hypereosinophilia, extensive cerebral infarction which was primarily affecting the border zone, focal intracerebral hemorrhaging and a negative cerebral angiographic result. The distinguishing feature of our case was the occurrence of focal intracerebral hemorrhaging, but a negative cerebral angiographic result, which has not previously been reported. The occurrence of focal intracerebral hemorrhaging may suggest direct endothelial injury or vasculitis by eosinophilic infiltration as well as a distant thromboembolism.
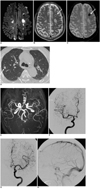
A 52-year-old man was admitted after a 1-month history of general weakness which was aggravated three days before an emergency room visit. An initial neurologic examination revealed disorientation of time, place, person, attention deficit, immediate memory disturbance, dysarthria, weakness of bilateral upper extremities with increased deep tendon reflex and right hemiparetic gate. The blood work noted an elevated eosinophil count (5,500/µL, 41% of whole white blood cells). The patient's clinical history and basic examinations did not lend a clue to any identifiable cause of hypereosinophilia, which includes parasitic infection, neoplasm, vasculitis or allergy. Additionally, the patient had no prior clinical symptoms or signs suggesting the occurrence of Churg-Strauss syndrome, which includes sinusitis or asthma. The cardiac enzymes were increased by 7.3 ng/ml of creatine kinase-MB and 4.78 ng/ml of troponin-I. An echocardiogram revealed mild left ventricular inferior wall hypokinesia. A diffusion-weighted brain MRI revealed multiple acute infarcts in bilateral border zones, as well as randomly distributed cortices (Fig. 1A). Focal intracerebral hemorrhaging was noted on the left frontal white matter on T2-weighted and gradient echo images (Fig. 1B, C). A chest CT revealed multiple nodules in both lungs (Fig. 1D). An MR angiography and conventional cerebral digital subtraction angiography did not show any evidence of arterial or venous sinus abnormalities (Fig. 1E-H).
Under the diagnosis of acute hypereosinophilic syndrome, treatment consisted of 60 mg/day of prednisolon and 100 mg/day of aspirin. Clinical improvement was noted on the third day of treatment and the patient was discharged after two weeks with a full recovery of his general condition and no neurologic deficit.
Idiopathic hypereosinophilic syndrome is defined by 1) persistent eosinophilia of 1,500/µL or greater for at least six months or death before six months with signs and symptoms of hypereosinophilic syndrome disease, 2) lack of evidence of parasitic, allergic, or other recognized causes of eosinophilia despite careful examination, and 3) signs and symptoms of organ system involvement (4). Our patient did not fulfill one of these criteria due to an acutely manifested symptom. Though there was no recognized cause of eosinophilia, the diagnosis of our case would be acute hypereosinophilia rather than idiopathic eosinophilic syndrome.
In our patient, the organ involvement of eosinophils resulted in cerebral infarcts, increased cardiac enzymes and multiple lung nodules. The cerebral infarcts were noted at the vascular border zone as well as randomly distributed cortices. The absence of prolonged systemic hypotension episodes and negative cerebral angiographic results may suggest distal arteriolar occlusion related to local thrombi or distant microemboli and spontaneous resolution. In a patient with hypereosinophilia, the source of thromboembolism is known to be the eosinophilic involvement of cardiac endocardium and cerebral arteriolar endothelium, because activated eosinophils injure human endothelial cells by releasing a number of cytopathic substances such as major basic protein (2). Major basic protein is a potent stimulator of platelet activation and aggregation which binds to thrombomodulin and reduces its ability to inhibit the clotting cascade (5). Cardiac endothelial injury is often extensive and results in fibrosis and thrombosis of endocardial surfaces, though it may be clinically silent at the early stages of disease (6). The thrombi made in the heart can induce cerebral infarcts or a pulmonary embolism. Elevated cardiac enzymes and mild left ventricular hypokinesia on the echocardiogram in our patient might be related to cardiac involvement of hypereosinophilia or coexisting ischemic heart disease. However, a specific diagnosis could not be made because further extensive cardiac examination was not available.
In cerebral arteries, both thromboembolic infarction and direct eosinophilic toxicity may be caused by hypereosinophilia. Microthromboemboli preferentially embolize along vessels to the border zones. This was proved by a prior experimental report and was related to the size of the embolic particles (7). Thus, as shown in our case, the cerebral infarctions are produced at the vascular border zone as well as randomly distributed cortices. The main mechanism of eosinophilic toxicity to cerebral arteriolar endothelial cells was thought to be through the production of the tumor necrosis factor (TNF)-α mediated by an eosinophil cationic granular protein and resultant multiple arteriolar fibrinocruoric thrombi (8).
Hypereosinophilia and its neurologic manifestations have been discussed in many previous reports. However, no report of a case with focal intracerebral hemorrhaging and angiographic demonstration of an intact cerebral vasculature has been reported. The focal intracerebral hemorrhaging in our patient was thought to be unusual because the individual infarct size was not large and no bleeding diathesis was observed. This may suggest that there could be direct endothelial injury or vasculitis caused by eosinophilic infiltration as well as distant thromboembolism.
Another possible mechanism of the border zone infarct is blood hyperviscosity with local thrombus formation. Both border zone infarcts in extreme hypereosinophilia, as well as hyperviscosity states from polycythemia vera have been reported (9, 10). However, even in those cases, the cause of cerebral infarcts is more likely a microthromboembolism related to a prothrombotic state rather than local arterial thrombosis, unless the gross arterial or venous obstructions were demonstrated on angiograms (10). Therefore, the latter scenario was not applicable to our patient.
This case suggests that multiple cerebral infarcts in the vascular border zone without a specific recognizable cause, as well as cerebral angiographic abnormalities, can be a cause of neurologic manifestation associated with hypereosinophilia. Though the infarct sizes are not extensive, intracerebral hemorrhaging may be accompanied with infarcts related to hypereosinophilia. Hence, in such cases, the administration of antithrombotic drugs is very important.
Figures and Tables
Fig. 1
Hypereosinophilia with multiple thromboembolic cerebral infarcts and focal intracerebral hemorrhaging in 52-year-old man.
A-C. Diffusion-weighted image (A) reveals multiple acute infarcts in bilateral border zones. T2-weighted image (B) and gradient echo image (C) reveal focal intracerebral hemorrhaging (arrows) at left frontal white matter.
D. Axial high resolution CT scan of lung reveals multiple nodules (arrows).
E-H. Cerebral MR angiography (E) and digital subtraction angiography (F-H) shows no evidence of arterial or venous sinus abnormalities.
References
1. Prick JJ, Gabreëls-Festen AA, Korten JJ, van der Wiel TW. Neurological manifestations of the hypereosinophilic syndrome (HES). Clin Neurol Neurosurg. 1988. 90:269–273.
2. Moore PM, Harley JB, Fauci AS. Neurologic dysfunction in the idiopathic hypereosinophilic syndrome. Ann Intern Med. 1985. 102:109–114.
3. Torvik A. The pathogenesis of watershed infarcts in the brain. Stroke. 1984. 15:221–223.
4. Chusid MJ, Dale DC, West BC, Wolff SM. The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine (Baltimore). 1975. 54:1–27.
5. Slungaard A, Vercellotti GM, Tran T, Gleich GJ, Key NS. Eosinophil cationic granule proteins impair thrombomodulin function. A potential mechanism for thromboembolism in hypereosinophilic heart disease. J Clin Invest. 1993. 91:1721–1730.
6. Sarazin M, Caumes E, Cohen A, Amarenco P. Multiple microembolic borderzone brain infarctions and endomyocardial fibrosis in idiopathic hypereosinophilic syndrome and in Schistosoma mansoni infestation. J Neurol Neurosurg Psychiatry. 2004. 75:305–307.
7. Pollanen MS, Deck JH. The mechanism of embolic watershed infarction: experimental studies. Can J Neurol Sci. 1990. 17:395–398.
8. Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990. 171:2025–2041.
9. McMillan HJ, Johnston DL, Doja A. Watershed infarction due to acute hypereosinophilia. Neurology. 2008. 70:80–82.
10. Koennecke HC, Bernarding J. Diffusion-weighted magnetic resonance imaging in two patients with polycythemia rubra vera and early ischemic stroke. Eur J Neurol. 2001. 8:273–277.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download