This article has been corrected. See "Erratum" in Volume 10 on page 651.
Abstract
Objective
To determine the utility of intercellular adhesion molecule (ICAM)-1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA-anti-ICAM-1) as a targeted contrast agent for the molecular magnetic resonance imaging (MRI) in collagen-induced arthritis (CIA).
Materials and Methods
Three groups of mice were used: non-arthritic normal, CIA mice in both the early inflammatory and chronic destructive phases. The MR images of knee joints were obtained before and after injection of Gd-DTPA-anti-ICAM-1, Gd-DTPA, and Gd-DTPA-Immunoglobulin G (Ig G) and were analyzed quantitatively. The patterns of enhancement on the MR images were compared with the histological and immunohistochemical ICAM-1 staining.
Results
The images obtained after injection of Gd-DTPA-anti-ICAM-1 displayed gradually increasing signal enhancement from the moment following injection (mean ± standard deviation [SD]: 424.3 ± 35.2, n = 3) to 24 hours (532 ± 11.3), rather than on pre-enhanced images (293 ± 37.6) in the early inflammatory phase of CIA mice. However, signal enhancement by Gd-DTPA and Gd-DTPA-IgG disappeared after 80 minutes and 24 hours, respectively. In addition, no significant enhancement was seen in the chronic destructive phase of CIA mice, even though they also showed inflammatory changes on T2-weighted MR images. ICAM-1 expression was demonstrated in the endothelium and proliferating synovium of the early inflammatory phase of CIA mice, but not in the chronic destructive phase.
Rheumatoid arthritis (RA) is a chronic inflammatory disease, and affected individuals experience significant morbidity, including loss of function, joint destruction, and permanent deformity, in addition to having a higher mortality than the general population (1). Molecular imaging techniques that use targeted agents binding specifically to inflamed synovium are increasingly allowing earlier diagnoses and assessment of a treatment response in RA (2-4). Several studies have shown that it is possible to target and visualize specific cellular processes in arthritic synovium using molecular imaging approaches (5-8). However, these studies are currently limited to nuclear and optical imaging methods in RA. In addition, magnetic resonance imaging (MRI) has not been used despite its particular advantage of simultaneously showing information at the anatomical and molecular levels.
The vascular endothelium is an attractive target for imaging and therapy in RA due to its obvious accessibility through the systemic circulation (4-6, 9). The intercellular adhesion molecule (ICAM)-1 is primarily found on synovial endothelial cells and helps recruit lymphocytes, monocytes, and neutrophils to the joints in RA (10-12). ICAM-1 also plays a role in the interactions between activated lymphocytes, fibroblast-like synoviocytes, and macrophage-like synoviocytes in the synovial lining and sublining (12-14). In addition, the immunohistochemical ICAM-1 findings were different for various stages of RA (15), and the potential therapeutic roles of anti-ICAM-1 agents have also been studied in several animal models of inflammatory arthritis and patients with RA (16-18). Thus, ICAM-1 represents a promising molecular target in specific targeted molecular imaging, drug delivery, and tissue engineering for patients with RA.
In a previous study, we produced ICAM-1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA-anti-ICAM-1) as an inflammatory cell-targeted MR contrast agent, which was prepared by bioconjugating Gd-DTPA and anti-ICAM-1. We confirmed that a molecular MRI using the Gd-DTPA-anti-ICAM-1 display targeted T1 MR images specific to ICAM-1 in the mouse abscess model (19). However, to date, no studies that utilize ICAM-1 as a candidate target for molecular MR imaging have been performed in the field of RA. Thus, the current study was designed to evaluate the utility of Gd-DTPA-anti-ICAM-1 as a targeted contrast agent for molecular MRI with a conventional 1.5 Tesla MR machine to diagnose and distinguish different stages of collagen-induced arthritis (CIA).
Bioconjugation of both the anti-ICAM-1 antibody and rat IgG (Sigma, St. Louis, MO) to diethylenetriamine pentaacetic acid bisanhydride (DTPABA) and gadolinium chloride (GdCl) was performed according to a previously described procedure (19). Briefly, the anti-ICAM-1 antibody was purified from the culture supernatant of rat hybridoma using protein A/G-coupled affinity chromatography. The anti-ICAM-1 antibody and rat IgG (control) were conjugated to DTPABA in PBS (pH 8.5) for 1 hour at room temperature. GdCl was dissolved in deionized water and added to the DTPA-antibody conjugate solution. One part of the DTPA-antibody complex was then combined with 40 parts of GdCl in 0.5 M sodium acetate (pH 5.5). The molar ratio of Gd to antibody was about 1:20 in quantitative analysis using ICP-MS (Agilent 7500A, Palo Alto, CA). The Gd-conjugated antibodies did not show any cytotoxic effect on the cultured endothelial cells. The calculated plasma half-life of the Gd-DTPA-antibody conjugate was 3.6 ± 0.16 hours, which is, in turn, ten times longer than that of Gd-DTPA (~20 min). The measured relaxivity (mmol-1sec-1) of the Gd-DTPA-anti-ICAM-1 (23.27 ± 1.31) was higher than that of Gd-DTPA (6.55 ± 0.58) as measured on a 1.5 Tesla MR scanner.
Male DBA/1J mice, 7-9 weeks of age and free of murine-specific pathogens, were purchased from Orient (Seoul, Korea), housed throughout the experiments in a laminar flow cabinet. To induce CIA, mice were immunized intradermally at the base of the tail with 100 µg of bovine type II collagen (CII; Chondrex, Redmond, WA) emulsified in an equal volume of complete Freund adjuvant (Chondrex). Three weeks later, the mice were boosted in the same manner. The day of the first injection of CII was considered day 0 for all mice. All animal procedures were conducted with approval from our Institutional Animal Care and Use Committee.
We used three groups of mice: normal non-arthritic mice (n = 3); mice in the early inflammatory phase of CIA (day 28, 4 weeks after 1st injection, n = 9); and mice in the chronic destructive phase of CIA (day 56, 8 weeks after 1st injection, n = 3). Prior to all MR imaging, all experimental mice were sedated by an intramuscular injection of 10 mg/kg of ketamine hydrochloride (Ketalar; Yuhan Yanghang, Seoul, Korea) in combination with 10 mg/kg of xylazine hydrochloride (Rumpun; Bayer Korea, Seoul, Korea). For MR imaging, T2-weighted fat-suppressed images were obtained before injecting contrast agents to evaluate the presence or absence of synovitis in the individual knee joints in all experimental mice. A T1-weighted dynamic MR imaging was then performed prior to and immediately after, as well as 10 minutes, 30 minutes, 80 minutes, 2 hours, and 24 hours after bolus injection of contrast agents into the tail vein. All nine experimental mice belonging to the early inflammatory phase of CIA group were analyzed, with three mice each receiving one of three types of MR contrast agents as follows: Gd-DTPA (100 nmoles of Gd/gm of body weight) as a non-targeted contrast agent, Gd-DTPA-anti-ICAM-1 (10 nmoles of Gd/gm of body weight) as a targeted contrast agent, and Gd-DTPA-IgG (10 nmoles of Gd/gm of body weight) as a control contrast agent. A T1-weighted dynamic MRI using Gd-DTPA-anti-ICAM-1 was also performed in the normal non-arthritic and chronic destructive phase of the CIA groups. In addition, plain radiographs were obtained to assess joint destruction using a full-field digital mammography system. Following the MRI, the mice were sacrificed by cervical dislocation to collect the knee joints for histopathologic and immunohistochemical analysis.
After sedation, the mice were placed in the prone position with their hind legs fixed to a plate with an adhesive bandage. All the in vivo MR images of CIA mice were obtained with a 1.5 Tesla clinical MR machine (Magnetom Symphony, Siemens Medical Systems, Erlangen, Germany). To maximize the signal-to-noise ratio, mice were placed with their knees below a small loop coil (diameter 5 cm) provided by the manufacturer. The fat-suppressed, coronal MR images were obtained before and after the intravenous injection of Gd-DTPA (Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, NJ), Gd-DTPA-IgG, and Gd-DTPA-anti-ICAM-1.
Imaging protocols for both the pre-contrast and post-contrast MR studies included the following sequences: fat-suppressed T1-weighted fast spin echo (FSE) (repetition time msec/echo time msec, 300/14; field of view, 42 × 56 mm; matrix dimension, 256 × 154), and fat-suppressed T2-weighted FSE (1700/103; echo train length, 15; field of view, 42 × 56 mm; matrix dimension, 256 × 154). The T1- and T2-weighted FSE images had the same sagittal positioning. The image sections were 2.0 mm thick for T1- and T2-weighted FSE images. An MRI was performed in the sagittal planes for all sequences.
For the quantitative analysis, signal intensity was measured on serial MR images using a region of interest (ROI) that was identified by two radiologists blinded to the grouping of the mice. One circular ROI with a diameter between 2 and 5 mm2 was placed in the same location of knee joints, and included high signal intensity areas, which were regarded as the synovial membrane, synovial fluid, and joints capsules. Discrepancies for the selection of ROIs were resolved by mutual consensus.
After the hind limbs were removed, the legs were fixed in formalin for radiographic imaging. The plain radiographs of the knee joints were obtained using a mammographic imager based on a direct detection flat panel array design (Mammomat NovationDR, Siemens Medical Solutions, Erlangen, Germany). A full field flat panel digital detector measuring 24 cm × 29 cm (maximum matrix size, 3328 × 4096; pixel size, 70 µm) was used. All images were obtained using exposure settings of 30 kVp and 90 mAs and taken with a 1.5X magnification.
The animals were sacrificed, and the knees were taken to be fixed in 10% neutral buffered formalin overnight and decalcified with Calci-Clear Rapid (Diagnostics, Atlanta, GA) overnight. The knees were embedded with paraffin, and the tissues were sliced into 5 µm sections for Hematoxylin and Eosin (H & E) as well as immunohistochemical staining. Anti-ICAM-1 polyclonal antibody (BD biosciences, San Jose, CA) was used for immunohistochemical staining.
The ICAM-1-targeted molecular MR images using Gd-DTPA-anti-ICAM-1 as a targeted contrast agent were examined in the early inflammatory phase of CIA mice (Fig. 1). First, T2-weighted fat-suppressed MR images of arthritic knee joints demonstrated synovial thickening and joint effusion as an area of hyper-intense signal in all mice in the early inflammatory phase of CIA. When pre- and post-enhanced T1-weighted images were compared, the quick T1 enhancement was shown immediately after injection of Gd-DTPA as a non-targeted contrast agent, which persisted for only a few minutes, and completely disappeared within 80 minutes in the arthritic knee (Fig. 1A). In contrast, dynamic MR images with Gd-DTPA-anti-ICAM-1 found the synovial enhancement to increase gradually and persist for 24 hours after injecting this ICAM-1-targeted contrast agent (Fig. 1B). Interestingly, although the Gd content of the Gd-DTPA-anti-ICAM-1 was almost ten times lower than that of Gd-DTPA, a significant T1 enhancement of inflamed synovium was observed in MR images with the Gd-DTPA-anti-ICAM-1. However, the dynamic MR images with Gd-DTPA-IgG, which was a Gd-conjugated control antibody with the same amount of Gd as the Gd-DTPA-anti-ICAM-1, showed signal enhancement for only 30-60 minutes. Moreover, this T1 enhancement disappeared within 24 hours (Fig. 1C). In addition, MR images in Figure 1 show well-preserved joints in the arthritic knees in all the early inflammatory phases of CIA mice.
Figure 2 reflects quite different patterns of the enhancement curves of these three kinds of contrast agents following the quantitative analysis of signal enhancement in the early inflammatory phase of CIA mice. T1 enhancement (mean ± standard deviation [SD]: 393.0 ± 49.1, n = 3) was demonstrated immediately after injecting Gd-DTPA (100 nmoles of Gd/gm of body weight), compared to pre-enhanced images (212.3 ± 35.4) and decreased T1 enhancement at 80 minutes (254.7 ± 20.2) in the early inflammatory phase of CIA mice. However, Gd-DTPA-anti-ICAM-1 (10 nmoles of Gd/gm of body weight) enhanced images display gradually increasing signal enhancement from immediately following injection (mean ± SD: 424.3 ± 35.2, n = 3) to 24 hours (532.0 ± 11.3) after injection of the targeted contrast agent compared to pre-enhance images (293 ± 37.6). Moreover, the MR images with Gd-DTPA-IgG show signal enhancement for only 30-60 minutes after injection of contrast agent compared to pre-enhance images (307 ± 19.7), which fades away within 24 hours (297 ± 7.6). The Gd-DTPA-IgG enhancement patterns of all three CIA mice, in the early inflammatory phase, were also similar, although the size of the synovial inflammatory area varied. In addition, the results of the quantitative analysis of signal enhancement were in accordance with the calculated plasma half-life of Gd-DTPA-antibody conjugate (3.6 ± 0.16 hours), which is ten times longer than that of Gd-DTPA (~20 minutes).
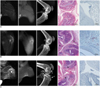
ICAM-1-targeted molecular MR images using Gd-DTPA-anti-ICAM-1 were also examined in the chronic destructive phase of CIA mice. Moreover, T2-weighted fat-suppressed MR images of arthritic joints demonstrated synovitis and joint destruction. However, very little to no signal enhancement was observed in arthritic knees up to 24 hours after injection of Gd-DTPA-anti-ICAM-1, compared with mice in the early inflammatory phase of CIA (Fig. 3). The patterns of enhancement curves following the quantitative analysis of signal enhancement of all three CIA mice in the chronic destructive phase were also similar (Fig. 4). In Figure 5, no signal enhancement was observed in the T1-weighted MR images of non-arthritic normal knee joints up to 12 hours after injection of Gd-DTPA-anti-ICAM-1. Also, plain film, H & E and immunohistochemical staining for ICAM-1 showed normal findings (Fig. 5A). The significant signal enhancement following the injection of Gd-DTPA-anti-ICAM-1 was noted in arthritic knee joints of early phase CIA mice (Fig. 5B). The joint of this early inflammatory phase of CIA mice demonstrated the marked inflammatory changes on T2-weighted MR images and marked synovial hyperplasia on H & E staining. The evidence of bone destruction was not observed on plain radiograph and H & E staining. Dense ICAM-1 expression is shown in the endothelium and is reduced in the subintimal layer of the proliferating synovium of mice in this early inflammatory phase of CIA. However, the ICAM-1-targeted synovial enhancement was not observed in the chronic destructive phase of CIA mice, even though they also showed inflammatory changes on T2-weighted MR images (Fig. 5C). Severe pannus invasion and bone destruction were observed on H & E staining and plain radiograph, but little to no ICAM-1 expression was observed in the synovium of the chronic destructive phase of CIA mice.
ICAM-1 is a promising target for the molecular imaging of RA. We demonstrated that Gd-DTPA-anti-ICAM-1 can be targeted to ICAM-1 that is expressed in the inflamed synovium of mice in the early inflammatory phase of CIA. Notably, this is the first study to identify the utility of targeted molecular MRI in the RA research field. We also showed that molecular MRI with Gd-DTPA-anti-ICAM-1 can differentiate the early inflammatory phase of CIA from the chronic destructive phase. Our observation shows the possibility of using Gd-DTPA-anti-ICAM-1 as a direct cellular targeting contrast agent in the molecular MRI for RA.
Monoclonal antibodies have limitations such as an increased circulating half-life and the human anti-mouse antibody response, although they have greater specificity for antigens as molecular imaging agents than conventional non-specific imaging agents. These shortcomings can produce increased background (blood pool) activity and may interfere with biospecificity (20). In our study, there are good reasons to conclude that the significant signal enhancement on MR images reflects the targeted ICAM-1 binding of Gd-DTPA-anti-ICAM-1 rather than both the unbound and the non-specifically bound fractions of this agent. First, on the MR images with Gd-DTPA-anti-ICAM-1, the T1 enhancement gradually increased and lasted for 24 hours (several times longer than the half-life of this ICAM-1-targeted contrast agent) in the arthritic joints of CIA mice. In contrast, T1 enhancement was shown immediately following the injection of Gd-DTPA as a non-targeted contrast agent, however lasted for only a few minutes. Also, the dynamic MR images with Gd-DTPA-IgG, Gd-conjugated control antibody with the same amount of Gd as the Gd-DTPA-anti-ICAM-1 demonstrated signal enhancement for only 30-60 minutes after the injection of contrast agent. Second, although the Gd content of the Gd-DTPA-anti-ICAM-1 was almost ten times lower than that of Gd-DTPA, a marked amount of T1 enhancement by this ICAM-1 targeted contrast agent was observed. Third, the signal enhancement by Gd-DTPA-anti-ICAM-1 on MR images and ICAM-1 expression on synovium, were observed in only the early inflammatory phase of CIA mice, but not in the chronic destructive phase. Thus, we concluded that prolonged signal enhancement on our MR images using Gd-DTPA-anti-ICAM-1 is a consequence of targeted binding to ICAM-1 in inflamed synovium.
Gd-DTPA is a non-specific contrast agent that is commonly used for MRI of hemodynamic parameters, including blood perfusion and vascular permeability (21). In contrast, Gd-DTPA-anti-ICAM-1 is a specific MR contrast agent that can be targeted to ICAM-1 and can thus identify specific cellular or molecular events that underlie disease. Gd-DTPA is transported in the plasma and diffuses freely throughout the interstitial space, but it is impermeable to the plasma membrane. In addition, it is excluded from cells and remains extracellular. This non-specific contrast agent accentuates differences in permeability or perfusion and is helpful in characterizing physiological processes, such as blood volume, flow, and perfusion (21). In RA, the inflamed synovium shows a large number of capillaries, enhanced capillary perfusion, and permeability. Thus, Gd-DTPA allows for the differentiation of the inflamed synovium from both surrounding healthy tissue and normal synovium through a marked increase of signal intensity on T1-weighted MR images (22-24). However, this contrast agent is nonspecific and does not provide any information regarding cellular and molecular mechanisms. Importantly, synovitis in RA is characterized by angiogenesis, cellular infiltration, and the production of various cytokines and adhesion molecules. While Gd-DTPA can only estimate blood perfusion and vascular permeability, Gd-DTPA-anti-ICAM-1 may enable the non-invasive evaluation of cellular and molecular changes, which often occur before any signs of pathologic and anatomical changes in the course of RA.
To our knowledge, this is the first report that identifies the utility and possible applicability of a targeted molecular MRI in the field of research for RA. Moreover, a conventional 1.5 Tesla MR machine, which is widely used in clinical practice, was used in our study. MRI has been regarded as a powerful imaging tool because of its noninvasive nature, high spatial resolution, and tomographic capabilities. In addition, MRI can visualize bone erosions much better than conventional radiography in the early stage of RA. Thus, MRI has been increasingly used to evaluate the joints of RA patients (25). The development of targeted MR contrast agents could dramatically expand the range of MR applications by combining the noninvasiveness and high spatial resolution of MRI with specific localization of molecular targets (21). Several successful attempts with various molecular targets and specific MR agents have been reported in the field of tumor imaging (26-29). However, MRI has not been used for studies using molecular imaging agents in RA, as these studies are currently limited to nuclear and optical imaging methods (3). The full MR imaging examination can be a lengthy procedure, and its low signal sensitivity has been a major limitation in experimental molecular imaging (24). Our study used Gd-DTPA-anti-ICAM-1, which has several unique characteristics including a higher conjugation ratio of Gd/antibody (1:20), a much longer plasma half-life (ten times longer than that of Gd-DTPA), and greater relaxitivity (two times higher than that of Gd-DTPA) as mentioned in a previous report (16). Therefore, a significant amount of T1 enhancement was obtained in the dynamic molecular MRI by overcoming the limitations of MRI as an experimental molecular imaging method. Limitations of our study include small sample size, no definite discrimination between synovial fluid and targeted synovium, and the absence of an ICAM-1 Ab-blocking study. Thus, further studies are needed to clarify the usefulness of molecular MRI using Gd-DTPA-anti-ICAM-1 as guidance for the ICAM-1 targeted diagnosis and a marker of therapeutic response in clinical practice.
In conclusion, this study demonstrates that Gd-DTPA-anti-ICAM-1 can be targeted to ICAM-1 expressed in the inflamed synovium during the early inflammatory phase of CIA mice. Thus, our results clearly show the feasibility of using Gd-DTPA-anti-ICAM-1 as a specific contrast agent for molecular MRI that targets ICAM-1 expressed in the inflamed synovium of CIA, the most reliable model of human RA. In RA, this novel tool may be used to evaluate pathophysiological processes, facilitate early diagnosis, and monitor therapy.
Figures and Tables
Fig. 1
MR images of knee joints in early inflammatory phase of collagen-induced arthritis mice. A. Gd-DPTA-injected; B. Gd-DPTA-anti-ICAM-1-injected; and C. Gd-DPTA-IgG-injected mice. In first column (far left), T2-weighted fat-suppressed MR images of arthritic knees of collagen-induced arthritis mice demonstrate thickened synovium and joint effusion as area of hyper-intense signal. Second column shows pre-contrast images; following columns (left to right) show dynamic T1-weighted MR images acquired immediately, 10 minutes, 30 minutes, 80 minutes, 2 hours, and 24 hours after injection of contrast agents. Quick T1 enhancement with Gd-DTPA was demonstrated immediately after injection and disappears after 80 minutes (A). MR images with Gd-DTPA-anti-ICAM-1 display gradually increasing signal enhancement in arthritic knee for 24 hours after injection of this targeted contrast agent (B). Signal enhancement with Gd-DTPA-IgG is observed for only 30-80 minutes after injection and disappears after 24 hours (C).

Fig. 2
Comparisons of mean enhancement curves in early inflammatory phase of collagen-induced arthritis mice injected with Gd-DTPA (n = 3), Gd-DTPA-anti-ICAM-1 (n = 3), or Gd-DTPA-IgG (n = 3) at different times.
Quick T1 enhancement (mean ± SD: 393.0 ± 49.1, n = 3) was demonstrated immediately after injecting Gd-DTPA (100 nmoles of Gd/gm of body weight) compared to pre-enhanced images (212.3 ± 35.4) and decreased at 80 minutes (254.7 ± 20.2). Gd-DTPA-anti-ICAM-1 enhanced images display gradually increasing signal enhancement from immediately (mean ± SD: 424.3 ± 35.2, n = 3) to 24 hours (532.0 ± 11.3) compared to pre-enhanced images (293 ± 37.6). MR images with Gd-DTPA-IgG show signal enhancement only for 30-60 minutes after injection and this T1 enhancement disappears within 24 hours.

Fig. 3
MR images with Gd-DTPA-anti-ICAM-1 of knee joint of mouse in chronic destructive phase of collagen-induced arthritis.
A. T2-weighted fat-suppressed MR image demonstrates synovitis and destruction of knee joints (arrows). B. Image obtained before injection. C-H. T1-weighted serial MR images obtained immediately, 10 minutes, 30 minutes, 1 hour, 2 hours, and 24 hours after Gd-DTPA-anti-ICAM-1 injection. Very little to no signal enhancement is observed in arthritic knee joints for up to 24 hours after injection of targeted contrast agent.
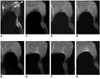
Fig. 4
Comparison of mean enhancement curves between early (n = 3) and chronic (n = 3) phases of collagen-induced arthritis in mice injected with Gd-DTPA-anti-ICAM-1 at different times. MR images obtained from mice in chronic destructive phase of collagen-induced arthritis, after injection of Gd-DTPA-anti-ICAM-1, show no significant signal enhancement for up to 24 hours.
CIA = collagen-induced arthritis

Fig. 5
MR images, plain films, as well as histopathological and immunohistochemical findings in A. non-arthritic normal mouse; B. early inflammatory phase; and C. chronic destructive phase of collagen-induced arthritis mice. In first column, T2-weighted fat-suppressed MR images show hyper-intense signals in arthritic joints in early inflammatory (B) and chronic destructive phases (C) of collagen-induced arthritis. In second column, ICAM-1-targeted T1-weighted MR images significant signal enhancement was demonstrated uniquely in mouse in early inflammatory phase of collagen-induced arthritis (B). In third column, only mouse in chronic destructive phase of collagen-induced arthritis showed bone erosion (arrows) under plain radiographs (C). Hematoxylin & Eosin staining (fourth column) shows synovial hyperplasia (asterisks) in early inflammatory phase of collagen-induced arthritis (B) as well as marked pannus invasion and bone destruction (arrowheads) in chronic destructive phase of collagen-induced arthritis (C). In last column, dense ICAM-1 expression is shown only in endothelium of proliferating synovium of mouse in early inflammatory phase of collagen-induced arthritis (B).
References
1. Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001. 44:1234–1236.
2. Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001. 219:316–333.
3. Wunder A, Straub RH, Gay S, Funk J, Muller-Ladner U. Molecular imaging: novel tools in visualizing rheumatoid arthritis. Rheumatology (Oxford). 2005. 44:1341–1349.
4. Garrood T, Pitzalis C. Targeting the inflamed synovium: the quest for specificity. Arthritis Rheum. 2006. 54:1055–1060.
5. Zinn KR, Chaudhuri TR, Smyth CA, Wu Q, Liu HG, Fleck M, et al. Specific targeting of activated endothelium in rat adjuvant arthritis with a 99mTc-radiolabeled E-selectin-binding peptide. Arthritis Rheum. 1999. 42:641–649.
6. Jamar F, Houssiau FA, Devogelaer JP, Chapman PT, Haskard DO, Beaujean V, et al. Scintigraphy using a technetium 99m-labelled anti-E-selectin Fab fragment in rheumatoid arthritis. Rheumatology (Oxford). 2002. 41:53–61.
7. Barrera P, van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van Lent PL, et al. Radiolabelled interleukin-1 receptor antagonist for detection of synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford). 2000. 39:870–874.
8. Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004. 50:2459–2465.
9. Koning GA, Schiffelers RM, Wauben MH, Kok RJ, Mastrobattista E, Molema G, et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006. 54:1198–1208.
10. Szekanecz Z, Haines GK, Lin TR, Harlow LA, Goerdt S, Rayan G, et al. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 1994. 37:221–231.
11. Suzuki M, Uetsuka K, Suzuki M, Shinozuka J, Nakayama H, Doi K. Immunohistochemical study on type II collagen-induced arthritis in DBA/1J mice. Exp Anim. 1997. 46:259–267.
12. Agarwal SK, Brenner MB. Role of adhesion molecules in synovial inflammation. Curr Opin Rheumatol. 2006. 18:268–276.
13. Tak PP, Thurkow EW, Daha MR, Kluin PM, Smeets TJ, Meinders AE, et al. Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol. 1995. 77:236–242.
14. Nakatsuka K, Tanaka Y, Hubscher S, Abe M, Wake A, Saito K, et al. Rheumatoid synovial fibroblasts are stimulated by the cellular adhesion to T cells through lymphocyte function associated antigen-1/intercellular adhesion molecule-1. J Rheumatol. 1997. 24:458–464.
15. Gerritsen ME, Kelley KA, Ligon G, Perry CA, Shen CP, Szczepanski A, et al. Regulation of the expression of intercellular adhesion molecule 1 in cultured human endothelial cells derived from rheumatoid synovium. Arthritis Rheum. 1993. 36:593–602.
16. Iigo Y, Takashi T, Tamatani T, Miyasaka M, Higashida T, Yagita H, et al. ICAM-1-dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J Immunol. 1991. 147:4167–4171.
17. Kakimoto K, Nakamura T, Ishii K, Takashi T, Iigou H, Yagita H, et al. The effect of anti-adhesion molecule antibody on the development of collagen-induced arthritis. Cell Immunol. 1992. 142:326–337.
18. Kavanaugh AF, Davis LS, Nichols LA, Norris SH, Rothlein R, Scharschmidt LA, et al. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994. 37:992–999.
19. Choi KS, Kim SH, Cai QY, Kim SY, Kim HO, Lee HJ, et al. Inflammation-specific T1 imaging using anti-intercellular adhesion molecule 1 antibody-conjugated gadolinium diethylenetriaminepentaacetic acid. Mol Imaging. 2007. 6:75–84.
20. Goldsmith SJ. Receptor imaging: competitive or complementary to antibody imaging? Semin Nucl Med. 1997. 27:85–93.
21. Delikatny EJ, Poptani H. MR techniques for in vivo molecular and cellular imaging. Radiol Clin North Am. 2005. 43:205–220.
22. Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Sonne-Holm S, Lorenzen I. Quantification of synovitis by MRI: correlation between dynamic and static gadolinium-enhanced magnetic resonance imaging and microscopic and macroscopic signs of synovial inflammation. Magn Reson Imaging. 1998. 16:743–754.
23. Peterfy CG. Magnetic resonance imaging of rheumatoid arthritis: the evolution of clinical application through clinical trials. Semin Arthritis Rheum. 2001. 30:375–396.
24. Cimmino MA, Innocenti S, Livrone F, Magnaguagno F, Silvestri E, Garlaschi G. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 2003. 48:1207–1213.
25. Boutry N, Morel M, Flipo RM, Demondion X, Cotten A. Early rheumatoid arthritis: a review of MRI and sonographic findings. AJR Am J Roentgenol. 2007. 189:1502–1509.
26. Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007. 13:95–99.
27. Gohr-Rosenthal S, Schmitt-Willich H, Ebert W, Conrad J. The demonstration of human tumors on nude mice using gadolinium-labelled monoclonal antibodies for magnetic resonance imaging. Invest Radiol. 1993. 28:789–795.
28. Konda SD, Aref M, Brechbiel M, Wiener EC. Development of a tumor-targeting MR contrast agent using the high-affinity folate receptor: work in progress. Invest Radiol. 2000. 35:50–57.
29. Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003. 63:2723–2727.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download