Abstract
Objective
To evaluate the effect of the heat-sink phenomenon induced by artificial ascites on the size of the ablation zone during percutaneous radiofrequency (RF) ablation of the hepatic subcapsular area in an in vivo rabbit model.
Materials and Methods
A total of 21 percutaneous rabbit liver RF ablations were performed with and without artificial ascites (5% dextrose aqueous solution). The rabbits were divided into three groups: a) control group (C, n = 7); b) room temperature ascites group (R, n = 7); and c) warmed ascites group (W, n = 7). The tip of a 1 cm, internally cooled electrode was placed on the subcapsular region of the hepatic dome via ultrasound guidance, and ablation was continued for 6 min. Changes in temperature of the ascites were monitored during the ablation. The size of the ablation zones of the excised livers and immediate complications rates were compared statistically between the groups (Mann-Whitney U test, Kruskal-Wallis test, linear-by-linear association, p = 0.05).
Results
One rabbit from the "W" group expired during the procedure. In all groups, the ascites temperatures approached their respective body temperatures as the ablations continued; however, a significant difference in ascites temperature was found between groups "W" and "R" throughout the procedures (39.2±0.4℃ in group W and 33.4±4.3℃ in group R at 6 min, p = 0.003). No significant difference was found between the size of the ablation zones (782.4±237.3 mL in group C, 1,172.0±468.9 mL in group R, and 1,030.6±665.1 mL in group W, p = 0.170) for the excised liver specimens. Diaphragmatic injury was identified in three of seven cases (42.9%) upon visual inspection of group "C" rabbits (p = 0.030).
The clinical role of percutaneous radiofrequency (RF) ablation for the treatment of small primary or secondary hepatic malignancies has been well established as both a complementary or alternative modality to surgery, as well as a first-line treatment (1-3). However, performing percutaneous RF ablations still involves some important issues to be resolved such as collateral thermal damage of the adjacent organs and considerable periprocedural pain. In cases where tumors are located in the far hepatic dome area, ultrasound (US)-guidance, which is the most widely used guidance method, is often limited. These drawbacks substantially restrict its clinical application.
Recently, a new technique utilizing artificially-infused ascites during the percutaneous RF ablation of hepatic tumors has been suggested as a solution to overcome these aforementioned drawbacks. This technique was proven to be effective at decreasing collateral thermal damage of the adjacent organs in an in vivo animal model (4), as well as demonstrating efficacy and safety in clinical trials (5, 6). The heat-sink phenomenon is another major obstacle to a successful RF ablation. Attributes of the heat-sink phenomenon caused by the major hepatic vasculatures during an RF ablation have been well known (7). They can distort the shape of the ablation zone and sometimes decrease the volume of the ablation zone (8). In the same sense, we thought that artificially-introduced fluid, which is usually colder than the body temperature, could lower the temperature of its surrounding, hence causing the heat-sink phenomenon. To the best of our knowledge, no study has been performed which has aimed to determine whether artificial ascites might cause the heat-sink phenomenon during a percutaneous RF ablation of the hepatic subcapsular area.
Thus, the purpose of this in vivo experiment is to evaluate whether or not the introduction of artificial ascites causes the heat-sink phenomenon, which in turn causes a decrease in the size of the ablation zone during a percutaneous RF ablation located in the hepatic subcapsular region. If this were the case, the next question would be whether or not the temperature elevation of artificial ascites can decrease or prevent the effect of the heat-sink phenomenon on the size of the ablation zone.
Our institutional animal care board, which had previously earned AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) accreditation, approved the protocol of this study. Twenty-one New Zealand white rabbits (average weight - 3.08 kg) were used for this study. For each experimental procedure performed, the study animals were under intravenous sedation using 5 mg/kg xylazine (Rompun; Bayer HealthCare, Leverkusen, Germany) through a 24-gauge, 1-inch intravenous catheter placed into the dorsal auricular vein after an intramuscular injection of 35 mg/kg ketamine hydrochloride (Ketalar; Yuhan Yanghang, Seoul, Korea). After an adequate degree of anesthesia was reached, we shaved the back and epigastric region of each rabbit for placement of an RF grounding pad (13 cm × 21 cm; Valleylab, Boulder, CO) in addition to a US examination, insertion of the RF electrode, and the sheathed needle, respectively. We placed the rabbits in the supine position on the grounding pad. The rabbits were randomly assigned to one of three groups by a predefined strategy. The groups were composed as follows: control group (group "C", n = 7); room temperature ascites group (group "R", n = 7); warmed ascites group (group "W", n = 7).
For each rabbit belonging to groups "R" and "W", we inserted a 20-gauge, 32-mm sheathed needle (Introcan Certo; B. Braun, Melsungen, Germany) into the gastrohepatic space via the subxiphoid or right subcostal approach under US guidance (Sequoia 512; Siemens Medical Solutions, Erlangen, Germany). We used a free-hand technique with an 8-12 MHz linear array transducer. After removal of the stylet, we drip-infused a 5% dextrose aqueous solution, which was initially at room temperature (for group "R"), and then warmed to 45℃ (for group "W"). The solution was added to the remaining sheath into the gastrohepatic space. The location of the needle tip in the gastrohepatic space could be assured when an enlarging anechoic area could be seen surrounding the liver contour with rapid drips of the solution. After confirming the location of the sheath tip, we switched to manual injection using 50 mL syringes in order to reduce the amount of time in which the artificial ascites would be within the peritoneal cavity. The injection was continued until a 1.0-1.5 cm separation between the liver and the diaphragm was achieved via US monitoring. The injected solution was defined as artificial ascites. After completing the injection, the wire thermocouple was inserted through the remaining sheath till the tip of the thermocouple was located in the middle of the artificial ascites, as visualized on a US image. This wire was sufficient to block regurgitation of the ascites through the sheath (Fig. 1).
We used an internally-cooled RF ablation system equipped with a 200-W RF generator and a single straight-tip electrode with a 1-cm active tip (Cool-tip; Valleylab). The tip was cooled with chilled water and the circulation of the cooling fluid was maintained with a pump (PE-PM Perfusion Pump; ValleyLab). We placed the tip of the RF electrode as close to the capsule of the hepatic dome of the right medial lobe or left median lobe under US guidance, as possible. The electrode placement for the rabbits belonging to groups "R" and "W" were performed after confirming the location of the sheath and before injecting the 5% dextrose aqueous solution. Following the creation of artificial ascites, the location of the electrode was reassured. The power of the generator was set to the maximum and maintained for 6 minutes (automatic mode). We maintained the initial location of the tip of the electrode over the course of the RF ablation under US guidance. We performed a total of one ablation per rabbit (Fig. 2). Moreover, one radiologist who had been performing US-guided RF ablations for six years created all of the artificial ascites and performed RF ablations.
We measured and recorded the body temperatures (anally) of each rabbit (before and after the RF ablation) using a penetration probe connected to one channel of a dual channel thermometer (Testo 922, Testo AG, Letzkirch, Germany). The temperatures of the artificial ascites before, during (at 3 minutes), and after ablation were recorded using a wire thermocoupler inserted via the sheath and connected to the other channel.
The rabbits were sacrificed immediately after each RF ablation procedure via the intravenous administration of a potassium chloride solution (10 mL). For the rabbits belonging to groups "R" and "W", we cautiously made a 1-2 cm incision in the epigastric region in order to avoid injuring any vessel. The artificial ascites were removed through this hole and we assessed the color of the fluid. Next, a midline incision was made from the xiphoid process to the umbilicus. The entire liver was exposed and excised and the abdominal cavity, including the peritoneal side of the diaphragm, was evaluated by visual inspection for any complications by the operator.
After excising the liver, we measured the longest (a) and largest perpendicular diameter (b) of the RF ablation zone on the capsule of the excised liver and calculated the area under the assumed ellipsoidal configuration (π × a × b / 4). We sectioned the liver along the longest diameter of the capsular ablation zone perpendicular to the capsule, and then, measured two perpendicular diameters (length and width; c, d) likewise. An additional cross-section was made perpendicular to the previous one, which enabled us to measure the dimension corresponding to thickness (e). We could then calculate the volume under the assumed oval configuration (π × c × d × e / 6). Each measurement was performed by another investigator taking into account only the white ablation zone.
The experimental data was analyzed using SPSS for Windows (version 11.0.1; SPSS Inc. Chicago, IL), with a significant p-value of 0.05. The body temperatures and the size of the ablation zones of each group of rabbits from all were compared using the Kruskal-Wallis test. The temperatures of the ascites for groups "R" and "W" rabbits were compared using the Mann-Whitney U test. Lastly, the frequencies of diaphragmatic thermal injury in the three groups were compared using a linear-by-linear association.
An artificial ascites was created successfully with a single puncture for rabbits belonging to groups "R" and "W". The volume of the artificial ascites injected ranged from 400-500 mL (mean - 430 mL). The additional time required for the preparation of artificial ascites ranged from 4 to 7 minutes (mean - 5.5 minutes).
One rabbit in group W expired after 3 minutes during the RF ablation procedure. A postmortem examination of that rabbit revealed bloody ascites due to hemoperitoneum. However, we could not identify the source of bleeding after a thorough examination. In addition, there was no evidence of peritoneal thermal injury. We excluded this rabbit from our study group because the complete protocol of the RF ablation procedure was not fulfilled. For all of the other rabbits from groups "R" and "W", the color of the artificial ascites was clear with no evidence of peritoneal thermal injury, diaphragmatic injury, and any other complication related to either the creation of the artificial ascites or the RF ablation. For three rabbits (42.9%) from group "C", diaphragmatic thermal injury was identified by a color change (p = 0.030). In these cases, only a color change on the peritoneal surface of the diaphragm was noted without evidence of perforation.
The baseline body temperatures, before the injection of the artificial ascites and RF ablations for the three groups, did not differ from each other (p = 0.182). However, the temperature changes, following an RF ablation on the rabbits belonging to group "R", were significantly larger than the changes for the other groups (p = 0.032) (Table 1).
The temperature of the 5% dextrose aqueous solution before injection into the group "W" and "R" rabbits were 46.6 ± 1.8℃ and 23.5 ± 1.9℃, respectively, and approached body temperatures after injection and as the RF ablations continued. However, the temperatures of the artificial ascites in the group "W" rabbits were significantly higher than the group "R" rabbits throughout the procedures (p < 0.05) (Table 2), which indicates that the two different temperature-dependent environments surrounding the RF ablation field were successfully attained.
After sacrificing the rabbits, we could identify the RF ablation zones on the hepatic capsule and on the cross-sectional surfaces of the liver by means of visual inspection and palpation in all cases. The area of the ablation-induced color change on the hepatic capsule was largest for the group "C" rabbits, followed by the groups "R" and "W"; however, no statistical differences were detected (p = 0.188). Similarly, the volumes of the lesions after cross sectioning did not reveal any significant differences among the three rabbit groups, either (p = 0.170) (Fig. 3) (Table 3).
Efforts to reduce collateral thermal injury of adjacent organs by infusing fluids into either the peritoneal or pleural cavity have been attempted by many investigators for about a decade (4, 5, 9-16). In addition, Rhim et al. (6) utilized artificial ascites in a percutaneous RF ablation for the treatment of hepatocellular carcinoma located in the hepatic dome and reported relatively large-series clinical results. The use of artificial ascites, in this particular study, was shown to be effective at improving the US-visibility of the hepatic tumors in addition to the electrode paths by displacing the liver downward as well as reducing the frequency of collateral thermal damage. In a recent study by Hinshaw et al. (5), the use of artificial ascites demonstrated an obvious effect by decreasing postprocedural pain. Therefore, the use of artificial ascites during percutaneous RF ablation therapy for hepatic subcapsular tumors may be deemed as an effective solution to three issues involving these treatment methods.
The heat-sink phenomenon is yet another well-known, important drawback of the RF ablation procedure for the treatment of hepatic tumors. It becomes particularly problematic when one treats a tumor located near a large blood vessel with a high flow velocity. This phenomenon reduces the size of the thermal ablation zone by taking heat away via a rapid stream of low-temperature blood. Although the ascitic fluid does not seem to flow as actively as blood in the vessels, it should keep moving as a result of convection, especially in the region adjacent to heating when the hepatic subcapsular area is treated. Consequently, this may have a role in depriving the ablation zone of the heat produced by the RF energy. Therefore, we hypothesized that a sort of heat-sink phenomenon, though it was not as great as flowing blood, could also be induced by artificial ascites.
Our previous study (4) demonstrated that the presence of artificial ascites did not significantly affect the size of the RF ablation zone at the subcapsular region of the liver, which is consistent with our current results. However, our study shows improvement from the previous study with respect to study design. First, we tried to evaluate the effect of different temperatures of the artificial ascites by adding the group "W" rabbits. We created this group with the intention of knowing whether the warming of ascites may decrease or prevent an ascites-induced heat sink phenomenon, if present. We warmed the fluid up to 45℃, which was a temperature established from a pilot study, and represents the highest temperature that did not cause any instability in the vital signs of the rabbits throughout the experimental period. Second, we tried to create a more standardized environment in terms of the temperatures of the artificial ascites. We measured the changes in temperature of the artificial ascites directly and adopted a manual injection method using a syringe instead of a drip infusion method. This reduced the time period during which the artificial ascites was affected by the body temperature in the peritoneal cavity. As a result, we created two distinct temperature-dependent, in vivo models of the artificial ascites. Third, we used a 5% dextrose aqueous solution instead of normal saline. A recent study (10) reported that an 5% dextrose aqueous solution was more desirable than normal saline as the material of the artificial ascites during an RF ablation because of its non-ionic and iso-osmolar properties, which could create a more insulated environment for the electrical field.
Although we adopted a manual injection method in this study, it is not recommended in the clinical application of artificial ascites because a drip infusion method seems to be more physiological and requires less manipulation despite taking a longer time. This method was adopted with the intention to foster a more controlled environment for the artificial ascites.
Only three cases of diaphragmatic injury were noted and belonged to the group "C" rabbits. Although we found a significant difference for the incidence of diaphragmatic injury between the other groups, the frequency of injury in this study (42.9%, 3/7), was definitely lower than our previous study (100%, 5/5) (4). We assume this difference might be caused by the timing of the pathological examination. We evaluated the diaphragmatic lesions by visual inspection immediately after the ablation procedures. Hence, very mild injury which appeared normal but could potentially cause an inflammation, followed by adhesion in the future may have been overlooked. Despite the differences in frequency between groups, and this is beyond the main interest of our study, the role of artificial ascites in preventing collateral thermal injury caused by percutaneous RF ablation of the hepatic subcapsular area was once again demonstrated.
In this study, we aimed to measure the temperature of the RF electrode after halting the operation of the cooling pump by means of the temperature display window, which was a feature of the RF generator, in order to determine the temperature of the ablation zone. However, in several cases, temperatures were temporally so unstable with high variation, that they were not deemed to be reliable and may not have reflected the real temperature of the lesion. We speculated that the instability was caused by the location of the electrode tip abutting the hepatic capsule. In some cases, an electrode tip might be able to penetrate the capsule and actually record the temperature of ascites. This kind of penetration could be identified in some of the excised liver specimens (Fig. 3A, B).
The present study does however have some limitations. First, although we measured the temperatures of the artificial ascites, the location of the measurement was at the gastrohepatic space which was not necessarily at the exact site where the heat-sink phenomenon may have occurred. We were unable to exclude the possibility of a small difference in the temperatures of the ascites between the two areas. Second, from a clinical point of view, the heat-sink effect is sometimes manifested as an obvious decrease in the size of the ablation zone, but more often than not, the presence of this effect is pointed out only by local tumor progression at the site where the tumor is abutted to a large vessel. In this case, survival of a microscopic residual tumor is thought to be responsible for this type of recurrence (17). We do not think our results can be applied to the heat-sink phenomenon at the microscopic level. This would have to be evaluated by a clinical study adopting this technique in the treatment of hepatic subcapsular malignancies with a long-term follow-up period. Third, we tried to standardize the locations of the electrodes. However, certain factors that could confound the size of the ablation zone, including different configurations of the liver contour and the major hepatic vessels around the ablation zone. Fourth, we did not perform a histopathological analysis for the ablated liver and diaphragm. This may be a source of error in the incidence of diaphragmatic injury. However, we do not think it significantly affected the estimated size of the liver ablation.
Despite these limitations, we successfully created different temperature-dependent in vivo models of artificial ascites and, from these models, we can conclude that the use of the artificial ascites technique during a percutaneous RF ablation in the subcapsular region of the liver does not induce any significant heat-sink phenomenon that can affect the size of the ablation zone. This conclusion is valid regardless of the temperature of the artificial ascites.
Figures and Tables
Fig. 1
Experimental setting which will determine effect of heat-sink phenomenon induced by artificial ascites during percutaneous radiofrequency ablation. Abdomen of New Zealand white rabbit is distended due to previously injected artificial ascites. 20-gauge, 32-mm sheath (arrow) with tip located in gastrohepatic space via right subcostal area and wire thermocouple (arrowheads) inserted through sheath lumen. Internally-cooled radiofrequency electrode was also inserted via epigastric abdomen.
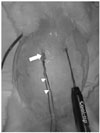
Fig. 2
US images of experimental setting before and after percutaneous radiofrequency ablation.
A. Shaft of radiofrequency electrode is shown as hyperechoic line (arrows), with its tip is barely noted (abutting hepatic capsule). Anechoic artificial ascites are filled in subphrenic peritoneal space separating liver (arrowheads) from diaphragm (open arrowheads) by more than 1.0 cm.
B. After applying radiofrequency energy for 6 minutes, hyperechoic change measuring about 1.5 cm is produced. Separation between (arrows) diaphragm and hepatic capsule is still identifiable.
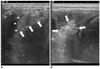
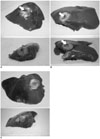
Fig. 3
Representative photographs of gross specimens from excised liver after radiofrequency ablation in groups "C" (A), "R" (B), and "W" (C). No significant difference is detected for either area on hepatic capsule or volume on cross section of radiofrequency-ablated zone. Small penetrations (arrows) of hepatic capsule made by tip of electrode are noted in some specimens (A, B).
References
1. Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007. 14:2319–2329.
2. Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007. 17:684–692.
3. Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005. 39:247–252.
4. Kim YS, Rhim H, Paik SS. Radiofrequency ablation of the liver in a rabbit model: creation of artificial ascites to minimize collateral thermal injury to the diaphragm and stomach. J Vasc Interv Radiol. 2006. 17:541–547.
5. Hinshaw JL, Laeseke PF, Winter TC 3rd, Kliewer MA, Fine JP, Lee FT Jr. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol. 2006. 186:S306–S310.
6. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008. 190:91–98.
7. Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002. 178:47–51.
8. Kim SK, Lim HK, Ryu JA, Choi D, Lee WJ, Lee JY, et al. Radiofrequency ablation of rabbit liver in vivo: effect of the pringle maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol. 2004. 5:240–249.
9. Kapoor BS, Hunter DW. Injection of subphrenic saline during radiofrequency ablation to minimize diaphragmatic injury. Cardiovasc Intervent Radiol. 2003. 26:302–304.
10. Laeseke PF, Sampson LA, Brace CL, Winter TC 3rd, Fine JP, Lee FT Jr. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol. 2006. 186:S249–S254.
11. Ohmoto K, Yamamoto S. Percutaneous microwave coagulation therapy using artificial ascites. AJR Am J Roentgenol. 2001. 176:817–818.
12. Ohmoto K, Tsuzuki M, Yamamoto S. Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol. 1999. 172:65–66.
13. Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003. 38:1066–1070.
14. Shibata T, Iimuro Y, Ikai I, Hatano E, Yamaoka Y, Konishi J. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002. 13:313–315.
15. Lee YR, Rhim H, Kim YS, Cho OK, Koh BH, Kim Y, et al. Intraperitoneal saline infusion during radiofrequency ablation of subcapsular hepatic tumor. J Vasc Interv Radiol. 2005. 16:753–754.
16. Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology. 2002. 222:819–823.
17. Kim YS, Rhim H, Lim HK, Park CK, Lee WJ, Do YS, et al. Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J Comput Assist Tomogr. 2006. 30:578–582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download