Abstract
Objective
To assess the clinical outcomes of the transcatheter microcoil embolization in patients with active lower gastrointestinal (LGI) bleeding in the small bowel, as well as to compare the mortality rates between the two groups based on the visualization or non-visualization of the bleeding focus determined by an angiography.
Materials and Methods
We retrospectively evaluated all of the consecutive patients who underwent an angiography for treatment of acute LGI bleeding between January 2003 and October 2007. In total, the study included 36 patients who underwent a colonoscopy and were diagnosed to have an active bleeding in the LGI tracts. Based on the visualization or non-visualization of the bleeding focus, determined by an angiography, the patients were classified into two groups. The clinical outcomes included technical success, clinical success (no rebleeding within 30 days), delayed rebleeding (> 30 days), as well as the major and minor complication rates.
Results
Of the 36 patients, 17 had angiography-proven bleeding that was distal to the marginal artery. The remaining 19 patients did not have a bleeding focus based on the angiography results. The technical and clinical success rates of performing transcatheter microcoil embolizations in patients with active bleeding were 100% and 88%, respectively (15 of 17). One patient died from continued LGI bleeding and one patient received surgery to treat the continued bleeding. There was no note made on the delayed bleeding or on the major or minor complications. Of the 19 patients without active bleeding, 16 (84%) did not have recurrent bleeding. One patient died due to continuous bleeding and multi-organ failure.
Conclusion
The superselective microcoil embolization can help successfully treat patients with active LGI bleeding in the small bowel, identified by the results of an angiography. The mortality rate is not significantly different between the patients of the visualization and non-visualization groups on angiography.
The definition of acute lower gastrointestinal (LGI) bleeding is bleeding with an origin distal to the ligament of Treitz. This phenomenon occurs at an average annual incidence rate of 20.5 per 100,000 patients (1). The therapeutic options for patients with massive LGI bleeding include surgery, vasopressin infusion, endoscopy, and transcatheter embolization. The results from past studies suggest that patients treated by surgery tend to have a high mortality rate (15-30%) (2); whereas, patients treated with vasopressin had high rates of complication and rebleeding (3). Traditionally, an endoscopy is the method of choice to investigate and treat LGI bleeding, yet it fails in 32% of cases, mainly because of the technical difficulties associated with the presence of blood clots, stool, and the amount of time required to prepare a patient for a colonoscopy (4, 5). In addition, sources of bleeding in the small bowel are not accessible via a colonoscopy.
The use of the transcatheter embolization has recently become a viable option for the management of LGI bleeding (6-13), especially if the angiography reveals the bleeding focus in the colon. Even though a patient shows clinical proof of active LGI bleeding, some patients do not show the bleeding focus on an angiography (14). To the best of our knowledge, only a few reports exist on treating LGI bleeding with a transcatheter microcoil embolization in the small bowel. Furthermore, even fewer clinical studies exist about comparing the mortality rates between the visualization versus the non-visualization groups when treating small bowel bleeding by transcatheter microcoil embolization.
The purpose of our study was to assess the clinical outcome after performing transcatheter microcoil embolizations in patients with active LGI bleeding, and comparing the mortality rates between groups of patients, defined visualization or non-visualization of the bleeding focus, respectively, as determined by an angiography.
We retrospectively evaluated the clinical records of all the consecutive patients, diagnosed with acute LGI bleeding and who underwent an angiography from January 2003 to October 2007. We obtained a waiver from the Institutional Review Board for patient consent for this study. In total, we identified 46 patients who underwent an angiography for LGI bleeding; however, we excluded 10 patients due to colonic bleeding with diverticulitis. The remaining 36 patients consisted of 25 men and 11 women (age range: 22 to 80 years, mean age: 62 years). These patients had clinically active, massive LGI bleeding as well as suspected small bowel bleeding, evidenced by active blood clots in the cecal area from the terminal ileum, and the inability to point out an active bleeding focus in the entire colon by a colonoscopy. We defined active LGI bleeding as hematochezia that occurred within 12 hours before an angiography. We considered massive bleeding to have occurred if the patients required a transfusion of at least four units of blood over a 12-hour period in the hospital, or the patients had hemodynamic instability (hypotension with a systolic blood pressure < 90 mmHg). We clinically monitored each patient after an angiography or embolization for any signs, symptoms or laboratory evidence of intestinal ischemia or recurrent intestinal hemorrhage. We performed a colonoscopy to identify active bleeding in 35 patients and an abdominal computed tomography (CT) in eight patients prior to an angiography. We found no evidence of an active bleeding focus on the CT.
We performed a right femoral artery puncture using the manual method, and a 6.0 Fr introducer sheath was inserted under fluoroscopic guidance. In addition, we performed a superior and inferior mesenteric angiography in each patient utilizing digital subtraction imaging and selective arterial contrast injections with standard 5-Fr catheters. We also performed the selective superior mesenteric artery angiography using different catheter locations under the following conditions: 15-30 ml of contrast media and a 300-500 psi injection pressure. This procedure evaluated the jejunal, ileal, and the ileocolic branches. After identifying a bleeding site, we performed a superselective embolization using a 3-Fr coaxial microcatheter system (Microferret; Cook, Bjaeverskov, Denmark) with a 0.016-inch guide wire (GT; Terumo, Tokyo, Japan). We positioned the microcatheter as close as possible proximal to the marginal artery or the vasa recta at the bleeding site. We used 3-2 mm or 4-2 mm microcoils (Tornado embolization microcoil: Cook, Bloomington, IN) as the embolic agents in all the patients. If the angiography did not show a bleeding focus, we performed a provocative test followed by an intraarterial injection of a vasodilator such as Alprostadil 1 ample (®Eglandin: WelFide Korea, Seoul, Korea) mixed with normal saline (30 cc). However, some patients who did not show a bleeding site on the mesenteric angiography underwent radionuclide scanning with technetium 99m-labeled red blood cells. If the radionuclide scan showed a suspected bleeding focus, we then performed a superselective angiography. If we identified a bleeding focus, we then performed an embolization. However, patients underwent conservative treatment if a radionuclide scan did not reveal a bleeding focus. We performed the embolizations close to the bleeding site and beyond the marginal artery or the vasa rectal level.
We analyzed the data according to the definitions and guidelines outlined for a percutaneous transcatheter embolization in the Society of Interventional Radiology (15). We defined technical success as the immediate cessation of bleeding as identified by a post-procedural angiography. We defined clinical success as the clinical cessation of bleeding and the stabilization of hemoglobin levels that required no more than 2U of packed red blood cells within 30 days after the procedure. We assessed the rebleeding by checking the clinical parameters passage of blood via the rectum and by a hematologic evaluation requiring more than 2U of packed red blood cells. We defined ischemic complications based on the outcome of this analysis. An example of a major ischemic complication was a bowel infarction that required surgery, whereas examples of minor ischemic complications were conditions that resulted in no sequelae and no further treatment.
We analyzed the outcomes and the clinical factors such as age, gender, cause of bleeding, hemodynamic support, presence of coagulopathy and mortality and subdivided the patients for the two subgroups: group A received an embolization at the bleeding site and group B did not receive undergo an embolization. If we were not able to identify a bleeding focus on an angiography and a patient had unstable vital signs, a drop of 2 mg or greater of hemoglobin levels after transfusion and/or if there was persistent melena, a surgical procedure was considered. We performed a Student's t or Chi-squared test (where appropriate) to determine if significant differences exist between the two groups and considered a p value less than 0.05 to be statistically significant.
The patient treatment algorithm is located in Figure 1. Upon the initial angiography, 14 patients (39%) had contrast extravasation in the LGI tract and consequently underwent a superselective transcatheter microcoil embolization (Fig. 2). Postembolization rebleeding occurred in 21% of these patients (3 of 14 patients) within one day of the embolization. Two patients showed active bleeding in the small bowel on radionuclide scanning with technetium 99m-labeled red blood cells and then underwent re-embolization using microcoils. One patient, however, continued to bleed (hemoglobin level dropped from 9 g/dL to 6 g/dL), and, as a result, underwent a surgical resection of the ileum. Following the resection, we discovered an ulcerative lesion in the ileum. Of the three patients with rebleeding, one with gastric cancer had continuous hematochezia that resulted in the death of the patient due to disseminated intravascular coagulation five days after embolization.
On the initial angiography, 22 patients did not show contrast extravasation. Next, we performed a provocative test on these 22 patients, which did not show any bleeding focus angiography. Next, 15 of the 22 patients underwent radionuclide scans due to continuous hematochezia. Seven of the 15 had a suspected bleeding focus in the small bowel and they subsequently underwent superselective reangiography of the superior mesenteric artery. Of the seven patients, three showed contrast extravasation or a pseudoaneurysm, and they underwent a superselective microcoil embolization. We did not observe the contrast extravasation on the final angiography.
For the 17 patients in group A, we performed a transcatheter embolization for the treatment of LGI bleeding in the small bowel, including three patients who underwent embolizations after performing a bleeding scan due to the negative findings on the initial angiography. The technical success rate was 100%; however, the complete clinical success was only 88% (15 of 17 patients). In contrast, we performed 19 superior mesenteric artery branch embolizations: ileocolic artery (n = 12), jejunal branch (n = 4) and ileal branch (n = 3). The angiography results revealed either contrast extravasation (n = 18) or a pseudoaneurysm (n = 1). We used 3-2 mm or 4-2 mm microcoils as a function of vessel size. Among all of the 17 patients who achieved complete clinical success, there were no clinical signs or symptoms of intestinal ischemia and no case of postembolic infarction. The mortality rate for the patients who received embolizations due to a bleeding focus was 6%.
The 19 patients belonging to group B underwent conservative management such as intravenous injections of vitamin K, vasopressin treatment for three days, and an intravenous packed red blood cell transfusion. Of the 19 patients 15 showed continuous hematochezia, which led to radionuclide scans. Although the radionuclide scans showed a suspected bleeding focus, four patients did not show additional contrast extravasation on the repeated angiography (Fig. 3). Of these four patients, three had continued bleeding. In addition, two of the patients had a history of taking non-steroidal anti-inflammatory drugs and consequently did not show the LGI bleeding a week later. One patient died due to continuous bleeding and multi-organ failure. The mortality rate for the patients without a bleeding focus visualized on an angiography was 5%.
The clinical data, including the laboratory findings, systolic blood pressure and number of transfusions before angiography for the two groups, revealed that the mortality rate was not significantly different between the two groups (Table 1).
The overall mortality rate for upper gastrointestinal (UGI) bleeding is approximately 14% (16), as opposed to less than 5% for LGI bleeding (1). Patients with LGI bleeding can present with various clinical conditions, which primarily characterized by minor bleeding that can be treated conservatively. Some patients have severe life-threatening intermittent bleeding or continual active bleeding. Patients with life-threatening bleeding or continual bleeding require examination by CT, colonoscopy, visceral angiography and/or radionuclide scans to identify the bleeding focus and administer the proper treatment.
The noninvasive diagnostic tools for investigating acute hematochezia by evaluating for the presence of a bleeding focus or the underlying disease include a CT or radionuclide scan (17, 18). Kuhle and Sheiman (17) suggested that the capability of the helical CT to depict acute LGI bleeding may exceed the lower bleeding limit of 0.5 mL/min, which was cited for the mesenteric angiography. Recent reports cite the use of a multidetector row helical CT for patients with acute massive gastrointestinal bleeding. The sensitivity and specificity were found to be above 90% (18). In our study, the CT finding did not show an active bleeding focus. Radionuclide scans can detect hemorrhaging rates as low as 0.1 mL/min (19). Diehl et al. (20) reported that 60% of patients (12 out of 20) had positive findings on radionuclide scans, and eight of the 12 patients underwent surgery. For patients with acute LGI bleeding and negative or nondiagnostic endoscopy or CT findings, radionuclide scans improved the overall rate of bleeding detection. In our study, 20% of patients (3 of 15) had matching radionuclide scan and angiographic findings.
An endoscopy is the first step in the diagnosis and management of UGI and LGI bleeding (4, 5). An emergency endoscopy for LGI bleeding may be difficult because stool or blood in the colon may cause an adequate inspection of the mucosa to be impossible. Although purging may clear up retained blood and clots, active bleeding frequently fills up the lumen and this limits the usefulness of the examination. Moreover, patients need to be hemodynamically stable and have undergone adequate colonic preparation to perform a colonoscopy (21). In addition, a bleeding source in the small bowel does not lend to easy access for endoscopic investigations. In our study, 97% of patients (35 of 36) underwent a colonoscopy for the diagnosis and management of LGI bleeding; however, we could not treat them because of the inability to perform an adequate inspection due to the active bleeding and the location of the source of the bleeding in the small bowel.
We identified 56 cases in the literature in which a transcatheter embolization was the treatment of LGI bleeding in the small bowel (Table 2) (6-12). The technical success rate ranged from 40-100%, and the rebleeding rate was 0-40%. We found two cases with major ischemic complications; one case of fistula formation and one case of ileal segmentectomy (6, 10). In our study, the technical success rate was 100% and the rebleeding rate was 21%. The choice of occlusive agents depended on the location of the transcatheter embolization and operator preference. Most recent investigators have used gelfoam, polyvinyl alcohol particles (PVA), N-butyl cyanoacrylate (NBCA), microcoils or some combination of these materials (22-24). We preferred to use microcoils ranging in size from 3-2 mm to 4-2 mm since they are easy to see, control, and deploy accurately. Smaller PVAs may reach the intramural circulation, while larger PVAs may fragment upon injection and collect at the submucosal plexus beyond the level of collateralization (22). Gelfoam has the disadvantage of being a temporary occlusive agent and it may cause an ischemic change in the intestinal wall after distal embolization (23). Jae et al. (24) recently reported that transcatheter arterial embolization with NBCA is a highly effective and safe treatment for UGI bleeding; especially if it is not possible to advance the microcatheter to the bleeding site or if the patient suffers with coagulopathy. However, there no reports exist on the clinical usefulness of NBCA in patients with LGI bleeding.
The localization of the bleeding site by angiographic evaluation requires the patient to be actively bleeding at the time of the study (14). An exhaustive investigational workup failed to identify a definitive bleeding site in some patients, which resulted in the patients to undergo repeated invasive investigations and blood transfusions. Blind surgical resections of the colon for patients with nonlocalized LGI bleeding were associated with a 33% mortality rate in one surgical series (25). For the localization of bleeding sites, provocative angiographic studies with the use of intraarterial tissue plasminogen activator (tPA), heparin and tolazoline have been reported (26, 27). Ryan et al. (26) reported that an intraarterial provocative mesenteric angiography with heparin, vasodilator, and tPA identified the site of bleeding in 38% of patients and that this contributed to the treatment for 50% of the patients. However, in our study, all the patients with no visualized bleeding focus underwent repeated angiography as well as provocative testing, and received conservative management. Only one patient (5%) died due to continuous bleeding and multi-organ failure. This mortality rate is not different then the mortality rate observed in the group that underwent embolization of the bleeding focus (6%). However, Burgess and Evans (14) reported that patients without active bleeding identified at the time of an angiogram had a proven ischemia rate of 60% and a 60% mortality rate from continued bleeding or intestinal ischemia. We thought that this difference in mortality rates compared to the current study might be due to differences of the underlying diseases for the study group.
Our study had some limitations; including the fact that it was a retrospective analysis and the sample size was small. As a result, the refinement and clinical validation of this method requires a prospective study. A prospective, randomized study may provide a more accurate evaluation of the treatments for LGI bleeding with information that is more succinct. Second, in our study, we partially diagnosed the minor ischemic changes of the small bowel depending on the patient's symptoms. However, the ischemic changes of the bowel require assessment by regular US and CT examinations.
In conclusion, the superselective microcoil embolization should be considered as a viable treatment modality for the treatment of patients with active LGI bleeding in the small bowel. The mortality rates are not significantly different between the patients of the visualization and non-visualization groups on angiography.
Figures and Tables
Fig. 1
Treatment modalities for 36 patients with lower gastrointestinal hemorrhaging. Tx = treatment, pts = patients
Fig. 2
Results from 81-year-old woman with acute lower gastrointestinal bleeding.
A. Selective arteriogram, obtained after coaxial advancement of catheter into right ileocolic artery, shows active hemorrhaging (arrow).
B. Branch of right ileocolic artery has been embolized with single 3-2 mm microcoil (arrow).
C. Postembolization arteriogram after superselective embolization shows total occlusion of feeding vessel and cessation of hemorrhaging.
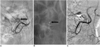
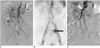
Fig. 3
Results from 69-year-old male patient with acute lower gastrointestinal bleeding following surgical treatment of gastric cancer.
A. Superior and inferior mesenteric arteriogram reveals no abnormal extravasation of contrast medium.
B. Radionuclide scan with technetium 99m-labeled red blood cells, performed immediately due to continued bleeding, displays bleeding focus (arrow) from increased isotope uptake in left lower quadrant.
C. Repeated angiogram does not show extravasation of contrast medium. Patient underwent conservative management and did not show any further bleeding one week later.
Acknowledgement
The authors thank Kevin Condren of the Harrisco Language Research Institute for his editorial assistance in the preparation and revision of the manuscript.
References
1. Longstreth GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1997. 92:419–424.
2. Billingham RP. The conundrum of lower gastrointestinal bleeding. Surg Clin North Am. 1997. 77:241–252.
3. Darcy M. Treatment of lower gastrointestinal bleeding: vasopressin infusion versus embolization. J Vasc Interv Radiol. 2003. 14:535–543.
4. Zuckerman GR, Prakash C. Acute lower intestinal bleeding: part I. Clinical presentation and diagnosis. Gastrointest Endosc. 1998. 48:606–617.
5. Zuckerman GR, Prakash C. Acute lower intestinal bleeding: part II. Etiology, therapy, and outcomes. Gastrointest Endosc. 1999. 49:228–238.
6. Gordon RL, Ahl KL, Kerlan RK, Wilson MW, LaBerge JM, Sandhu JS, et al. Selective arterial embolization for the control of lower gastrointestinal bleeding. Am J Surg. 1997. 174:24–28.
7. Peck DJ, McLoughlin RF, Hughson MN, Rankin RN. Percutaneous embolotherapy of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 1998. 9:747–751.
8. Defreyne L, Vanlangenhove P, De Vos M, Pattyn P, Van Maele G, Decruyenaere J, et al. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001. 218:739–748.
9. Bandi R, Shetty PC, Sharma RP, Burke TH, Burke MW, Kastan D. Superselective arterial embolization for the treatment of lower gastrointestinal hemorrhage. J Vasc Interv Radiol. 2001. 12:1399–1405.
10. Waugh J, Madan A, Sacharias N, Thomson K. Embolization for major lower gastrointestinal haemorrhage: five-year experience. Australas Radiol. 2004. 48:311–317.
11. d'Othee BJ, Surapaneni P, Rabkin D, Nasser I, Clouse M. Microcoil embolization for acute lower gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2006. 29:49–58.
12. Charbonnet P, Toman J, Buhler L, Vermeulen B, Morel P, Becker CD, et al. Treatment of gastrointestinal hemorrhage. Abdom Imaging. 2005. 30:719–726.
13. Luchtefeld MA, Senagore AJ, Szomstein M, Fedeson B, Van Erp J, Rupp S. Evaluation of transarterial embolization for lower gastrointestinal bleeding. Dis Colon Rectum. 2000. 43:532–534.
14. Burgess AN, Evans PM. Lower gastrointestinal haemorrhage and superselective angiographic embolization. ANZ J Surg. 2004. 74:635–638.
15. Drooz AT, Lewis CA, Allen TE, Citron SJ, Cole PE, Freeman NJ, et al. Society of Cardiovascular and Interventional Radiology. Quality improvement guidelines for percutaneous transcatheter embolization: SCVIR Standards of Practice Committee. J Vasc Interv Radiol. 1997. 8:889–895.
16. Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995. 311:222–226.
17. Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003. 228:743–752.
18. Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006. 239:160–167.
19. Gupta S, Luna E, Kingsley S, Prince M, Herrera N. Detection of gastrointestinal bleeding by radionuclide scintigraphy. Am J Gastroenterol. 1984. 79:26–31.
20. Diehl SJ, Ko HS, Dominguez E, Kaare Tesdal I, Kähler G, Böhm C, et al. Negative endoscopy and MSCT findings in patients with acute lower gastrointestinal hemorrhage. Value of (99m) Tc erythrocyte scintigraphy. Radiologe. 2007. 47:64–70.
21. Angtuaco TL, Reddy SK, Drapkin S, Harrell LE, Howden CW. The utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single center. Am J Gastroenterol. 2001. 96:1782–1785.
22. Kusano S, Murata K, Ohuchi H, Motohashi O, Atari H. Low-dose particulate polyvinyl alcohol embolization in massive small artery intestinal hemorrhage. Experimental and clinical results. Invest Radiol. 1987. 22:388–392.
23. Han YM, Lee JM, Jin KY, Lee SY, Kim CS. Embolization of superior mesenteric artery branches in dogs. Ischemic bowel changes depend on location of vessel occlusion and embolic materials. Invest Radiol. 1999. 34:629–635.
24. Jae HJ, Chung JW, Jung AY, Lee W, Park JH. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J Radiol. 2007. 8:48–56.
25. Setya V, Singer JA, Minken SL. Subtotal colectomy as a last resort for unrelenting unlocalized, lower gastrointestinal hemorrhage: experience with 12 cases. Am Surg. 1992. 58:295–299.
26. Ryan JM, Key SM, Dumbleton SA, Smith TP. Nonlocalized lower gastrointestinal bleeding: provocative bleeding studies with intraarterial tPA, heparin, tolazoline. J Vasc Interv Radiol. 2001. 12:1273–1277.
27. Johnston C, Tuite D, Pritchard R, Reynolds J, McEniff N, Ryan JM. Use of provocative angiography to localize site in recurrent gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2007. 30:1042–1046.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download