Abstract
Objective
We wanted to assess the long-term results of cyst ablation with using N-butyl cyanoacrylate (NBCA) and iodized oil in patients with autosomal dominant polycystic kidney disease (ADPKD) and symptomatic cysts.
Materials and Methods
Cyst ablation using a mixture of NBCA and iodized oil was performed in 99 cysts from 21 patients who had such symptoms as abdominal distension and pain. The collapse or reaccumulation of the ablated cysts after the procedure was assessed during the follow-up period of 36 to 90 months. The treatment effects, including symptom relief, and the clinical data such as the blood pressure and serum creatinine levels were also assessed, together with the complications.
Results
The procedure was technically successful in all 99 cysts from the 21 patients. Any procedure-related significant complications were not detected. Seventy-seven of 99 cysts (78%) were successfully collapsed on the follow-up CT. Twenty-two cysts showed reaccumulation during long-term follow-up period. The clinical symptoms were relieved in 17 of the 21 patients (76%). Four of 12 patients (33%) with hypertension and two of six patients (33%) with azotemia were improved. End stage renal disease (ESRD) occurred in six of the 21 patients (28%) during the follow-up period. The mean age of ESRD in our patients was 57 years. The mean time interval for the development of ESRD was 19 months.
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most frequent inherited disorders, and this is mainly characterized by innumerable cysts in the both kidneys, with or without the presence of cystic lesions in other organs (1-4). It is also the most frequent genetic cause of end stage renal disease (ESRD) in adults (3-9).
The progressive enlargement of renal cysts in ADPKD according to age can frequently cause abdominal distension or pain, hypertension, infection and hemorrhage of the urinary tract, nephrolithiasis and non-specific gastrointestinal manifestations such as nausea. The most common complication of the patients with ADPKD is abdominal distension or pain (10, 11).
When medical analgesics fail to relieve abdominal distension or pain, then decompressing procedures for the cysts may be necessary, including percutaneous aspiration followed by instillation of sclerosing agents, or surgical treatments such as laparoscopical decortication or marsupialization (12-16). The various sclerosing agents have traditionally been used for the ablation of benign renal cysts and to prevent their recurrence. We have previously reported the successful preliminary results of cyst ablation with using a mixture of n-bultyl cyanoacrylate (NBCA) and iodized oil in ADPKD patients.
The purpose of this study was to assess the long-term results of cyst ablation with using a mixture of NBCA and iodized oil, including the imaging and clinical data.
Between October 1999 and March 2008, 21 ADPKD patients with a total of 99 cysts were enrolled in this study. The study groups consisted of nine men and 12 women with a mean age of 51 years (age range: 33-78). We retrospectively reviewed the radiologic findings and medical records. The Institutional Review Board at our hospital approved this study.
Two radiologists (each with 10 years experience with various interventional genitourinary procedures) independently performed cyst ablation for the patients with ADPKD.
The treatment indications were as follow. All the patients complained of abdominal distension and/or pain that was caused by enlarged renal cysts, but none of them had received previous surgical treatment. When the analgesics failed to relieve the abdominal distension or pain, then cyst ablation was tried. The size of the cysts was a mean of 5-6 cm in diameter. A cyst that caused irritation to the adjacent peritoneum was also indicated for ablation. Twelve patients had hypertension (i.e., a systolic/diastolic pressure > 120/80 mmHg) and six patients had azotemia (i.e., an increased serum creatinine level > 1.4 mg/dL).
Because there was risk of leakage of the infected fluid or blood from the cyst during procedure, the patients who had acute symptoms such as cyst infection or bleeding were excluded from this study. All the patients underwent computed tomography (CT) for determining the indications and for targeting the cysts before the ablation. The measured maximal diameter of the cysts before the procedure was 3-10 cm (mean size: 6.5 cm).
Informed consent for the ablation was obtained for all the cases. The procedures were performed with the patient in either the prone or recumbent position after confirming the location of the targeted cyst by using ultrasonographic (US) guidance. After injection of local anesthesia, a 22-gauge puncture needle was inserted into the targeted cyst. The cystic fluid was aspirated as completely as possible and the fluid volume was measured.
After injection of 0.3 mL of 5% dextrose water, a mixture of 0.5 mL of NBCA and 1 mL of iodized oil (a ratio of 1:2) was injected regardless of the volume of the aspirated fluid. The plain radiograph was then obtained to ensure whether the injected materials were confined within the cyst. Then the same procedure was performed for the next cyst. The number of cyst ablations at a single session in a patient ranged from three to six.
The average follow-up period was 54 months (range: 36-90). Follow-up CT was performed for all the patients. The average time interval between the ablation and the first CT follow-up was at 36-80 months (mean interval: 45.3 months). CT follow-up was performed one to three times for each patient (mean: 2.3). CT follow-up was performed two times for 11 patients and three times for eight patients.
We assessed the rates of technical success and the immediate complications of the procedure. Technical success was regarded as obtainment of a collapsed cyst with opacity that represented the mixture of NBCA and iodized oil.
To analyze the long term results, we assessed the follow-up CT findings and we compared the clinical data of the patients. We categorized the long term results into total collapse, partial collapse and reaccumulation. We regarded as total collapse as < 10% volume of the initial volume, partial collapse as 10-50% volume of the initial volume and reaccumulation as > 10% volume of the initial volume or wash-out of the mixture of NBCA and iodized oil. Early reaccumulation was regarded as a collection of fluid in the ablated cyst on the first follow-up CT, and delayed reaccumulation was regarded as a collection of fluid in the ablated cyst on the second and third follow-up CTs.
We assessed the clinical relief of symptoms and the long term change of the clinical data of the patients, including the blood pressure and the serum creatinine levels. The rate of ESRD and the age this occurred were also assessed.
The volume of aspirated cystic fluid ranged from 10 to 300 mL (mean volume: 61 mL). The procedure was technically successful for all 99 cysts of the 21 patients. For nine cysts of four patients, two sessions of ablations were performed for the subsequent reduction of the cystic volume. On the radiograph obtained after the procedures, the radiopacity of the mixture of NBCA and iodized oil was correctly confined within all the injected cysts, without leakage to other organs. The eight patients complained mild pain immediately after the procedure. Any significant complications related to the procedures were not found.
The results of cyst ablation in this study are summarized in Table 1. During the follow-up period, 77 of 99 cysts (78%) were successfully ablated (Fig. 1) without reaccumulation of fluid. Forty-five of 77 cysts (58%) were totally collapsed and 32 cysts (42%) were partially collapsed. Eight of 99 cysts (8%) showed-up CT reaccumulation of fluid on the first follow-up CT, and 14 of 99 cysts (14%) showed delayed reaccumulation on the second and third follow-up CTs (Fig. 2). For the cysts that totally or partially collapsed, the mean diameter of the radiopacities that represent the mixture of NBCA and iodized oil was 2.3 cm. The mean diameter of the cysts with reaccumulation was 5.9 cm.
In 17 of 21 patients (76%), the symptoms such as abdominal distension or pain were relieved, while in the remaining four patients, their symptoms were not relieved or they were aggravated after the procedure. The effect of symptom relief was prolonged for 26 to 50 months (mean: 28.5).
All 12 patients with hypertension underwent medical treatment. Four of the 12 patients (33%) with hypertension became normotensive after the procedure, and this was prolonged for at least 36 months. Two of the six patients (33%) with azotemia had their serum creatinine level drop to the normal range, and this was prolonged for at least 19 months. ESRD occurred in six of 21 patients (28%) and this led to dialysis during the follow-up period. The mean age for the occurrence of ESRD in our patients was 57 years (age range: 52-66). The mean time interval between the initial cyst ablation and the presence of ESRD was 19 months (range: 15-24).
For the reduction of the cystic volume and to prevent cyst recurrence, ablation by instillation of sclerosing agents can be effectively used to destroy the cyst wall epithelium (17-19). Several agents have been used for this, including absolute ethanol, minocycline hydrochloride, povidoneiodine, acetic acid, ethanolamine oleate and holmium-166-chitosan complex. Of these various sclerosing agents, absolute ethanol is most commonly used (20-27). Absolute ethanol has generally been used as a safe and effective sclerosing agent in benign renal cysts. However, ablation by absolute ethanol instillation through a catheter is associated with various complications, including pain, fever and systemic reactions such as drunkenness and shock (25-27).
Especially, the application of absolute ethanol ablation is not easy in symptomatic patients with ADPKD because of the presence of multiple large cysts and the difficulty in identifying the cysts that are associated with symptoms (26, 27). Inserting the catheter into many cysts for ethanol ablation is not easy and this may make the patient uncomfortable, and ethanol ablation of a renal cyst usually takes several days (27).
N-butyl cyanoacrylate has recently been used for the embolization of vascular lesions in various parts of the body and for endoscopic management of bleeding and fistula (28). In contrast with absolute ethanol ablation, this procedure is simple without catheter insertion and this can be performed for multiple cysts in a single session.
No significant complications related with procedures using NBCA have been reported. Leakage of NBCA does not occur because of the immediate conversion of polymerize NBCA into a solid substance in ionic solutions such as tissue fluid and blood (27, 29).
Kim et al. (29) reported that percutaneous needle aspiration and intracystic injection of a mixture of NBCA with iodized oil were effective in reducing the volume of cysts and obtaining symptom relief in patients with ADPKD. However, that previous study had some limitations such that the reaccumulation of fluid in ablated cysts, the duration of symptom relief and the changes of such clinical data as the blood pressure and the serum creatinine level were not assessed on long-term follow-up.
Our study had a 100% technical success rate. Seventy-eight percent of the cysts were totally or partially collapsed without reaccumulation of fluid during the long term follow-up. In our study, the success rate was similar to the success rate for those patients who underwent ablation using absolute ethanol or other sclerosing agents for treating large simple renal cysts in other reports (25-27). The mixture of NBCA and iodized oil fixed the epithelial lining cells and caused permanent obliteration of the cyst lining.
Reaccumulation of the cystic fluid occurred in 22% of the ablated cysts. The reaccumulation rate was lower compared with the recurrence rate (32%) for ablation with using absolute ethanol in a two-year follow-up study (27). Reaccumulation usually occurred in large cysts and this might have been caused by the incomplete aspiration of the cystic fluid and the small amount of NBCA to cover the total area of the cyst epithelium. To avoid this, the needle tip should be monitored with US so that it remains in the center of the cyst during aspiration. Once the cyst fluid has been completely aspirated with a 10-mL syringe, we performed further repeated aspiration with using a 2-mL syringe. Nevertheless, ablation with using a mixture of NBCA and iodized oil might collapse almost all the large cysts in a single session without reaccumulation.
The symptoms were relieved in 76% of the patients during the follow-up period. The duration of subjective symptom relief was a mean of 28.5 months for 17 patients. Lee and Lee (27) reported that the probability of being pain free one year after absolute ethanol ablation was 64%, and this was in line with the results of our study. Although the patients' other cysts gradually grew, the symptoms did not develop again after ablation of the largest symptomatic cysts. Thus, selective cyst ablation may be an effective method for managing abdominal distension or pain in patients with ADPKD.
After the procedure, the four patients with hypertension and the two patients with azotemia were improved during more than 36 months follow-up. However the renal function of six patients, including four patients whose symptoms were relieved after the cyst ablation, decreased gradually and this resulted in ESRD during the follow-up period.
The development to ESRD has been shown to be highly variable in patients with ADPKD. In most patients, the renal function is maintained within the normal ranges, despite of the progressive growth of cysts, until the fourth to six decade of life (1-3). The average age and rate of development to ESRD in our study were not so different from the mean age and rate of developing to ESRD in untreated patients (3). Several studies have reported that patients with radiographically identifiable ADPKD have a 2% chance of developing ESRD by the age of 40 years, a 23% chance by the age of 50 and a 48% chance by the age of 73 (2, 3). Several studies have also shown that the kidney and cyst volumes are the strong predictors of renal function decline (1, 3). In all our patients with ESRD, other innumerable cysts were progressively increased in size, without regard to the collapse or reaccumulation of the ablated cysts. This study revealed only a limited effect of cyst ablation to prevent ESRD in patients with ADPKD. However we think that cyst ablation may be more effective to prevent ESRD if it is performed in the early stage of the ADPKD in a patient with only several large cysts.
This study had several limitations. This was a retrospective study and the image follow-up was not performed according to a regular time interval and protocol. Comparison of the clinical data of our patients with those of a control group was not performed. The indication criteria for cyst ablation were not well established. We could not analyze the results according to the extent and severity of ADPKD, which might have affected the clinical data in this study. We did not perform repeated cyst ablation for the treatment of any patients with recurrent hypertension or azotemia.
Despite the several limitations of this study, we believe that cyst ablation with using a mixture of NBCA and iodized oil may be a useful and safe method to obtain symptomatic relief in patients with ADPKD.
Figures and Tables
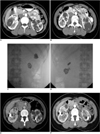
Fig. 1
36-year-old woman with autosomal dominant polycystic kidney disease.
A, B. Axial contrast enhanced CT scans show multiple cysts in both kidneys. Cyst ablation was performed for two cysts in right kidney and for two cysts in left kidney (asterisk).
C, D. Antero-posterior radiographies after procedure show lobulated radiopacities that represent mixture of N-butyl cyanoacrylate and iodized oil in right (C) and left (D) kidneys.
E. Follow-up CT scans 36 months after procedure show collapsed cysts with mixture of N-butyl cyanoacrylate and iodized oil.
F. Follow-up CT scans at 60 months show no evidence of reaccumulation in ablated cysts.
References
1. Schwenger V, Zeier M. Autosomal dominant polycystic kidney disease. Saudi J Kidney Dis Transpl. 1999. 10:7–20.
2. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993. 329:3323–3342.
3. Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic disease. Lancet. 2007. 369:1287–1301.
4. Segal AJ, Spataro RF. Computed tomography of adult polycystic disease. J Comput Assist Tomogr. 1982. 6:777–780.
5. Choyke PL. Inherited cyst disease of the kidney. Radiol Clin North Am. 1996. 34:925–946.
6. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002. 167:21–23.
7. Hayden CK Jr, Swischuk LE. Renal cystic disease. Semin Ultrasound CT MR. 1991. 12:361–373.
8. Hartman DS. Renal cystic disease in multisystem conditions. Urol Radiol. 1992. 14:13–17.
9. Romao EA, Moyses Neto M, Teixeira SR, Muglia VF, Vieira-Neto OM, Dantas M. Renal and extrarenal manifestations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006. 39:533–538.
10. Bennett WM, Elzinga L, Golper TA, Barry JM. Reduction of cyst volume for symptomatic management of autosomal dominant polycystic kidney disease. J Urol. 1987. 137:620–622.
11. Elzinga LW, Barry JM, Torres VE, Zincke H, Wahner HW, Swan S, et al. Cyst decompression surgery for autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1992. 2:1219–1226.
12. Pirson Y. Recent advances in the clinical management of autosomal-dominant polycystic kidney disease. QJM. 1996. 89:803–806.
13. Bajwa ZH, Gupta S, Warfield CA, Steinman TI. Pain management in polycystic kidney disease. Kidney Int. 2001. 60:1631–1644.
14. Wilson PD, Guay-Woodford L. Pathophysiology and clinical management of polycystic kidney disease in woman. Semin Nephrol. 1999. 19:123–132.
15. Gibson P, Watson ML. Cyst infection in polycystic kidney disease: a clinical challenge. Nephrol Dial Transplant. 1998. 13:2455–2457.
16. Chapman AB, Thickman D, Gabow PA. Percutaneous cyst puncture in the treatment of cyst infection in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1990. 16:252–255.
17. Hanna RM, Dahniya MH. Aspiration and sclerotherapy of symptomatic simple renal cysts: value of two injections of sclerosing agents. AJR Am J Roentgenol. 1996. 167:781–783.
18. Fontana D, Porpiglia F, Morra I, Destefanis P. Treatment of simple renal cysts by percutaneous drainage with three repeated injection. Urology. 1999. 53:904–907.
19. Chung BH, Kim JH, Hong CH, Yang SC, Lee MS. Comparison of single and multiple session of percutaneous sclerotherapy for renal cyst. BJU Int. 2000. 85:626–627.
20. Seo TS, Oh JH, Yoon Y, Lim JW, Park SJ, Chang SG, et al. Acetic acid as a sclerosing agent for renal cysts: comparison with ethanol in follow-up results. Cardiovasc Intervent Radiol. 2000. 23:177–181.
21. Uemasu J, Fugihara M, Munemura C, Nakamura E, Kawasaki H. Cyst sclerotherapy with minocycline hydrochrolide in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1996. 11:843–846.
22. Peyromaure M, Debre B, Flam TA. Sclerotherapy of a giant renal cyst with povidone-iodine. J Urol. 2002. 168:2525.
23. Kim JH, Lee JT, Kim EK, Won JY, Kim MJ, Lee JD, et al. Percutaneous sclerotherapy of renal cysts with a beta-emitting radionuclide, holmium-166-chitosan complex. Korean J Radiol. 2004. 5:128–133.
24. Bean WJ. Renal cysts: treatment with alcohol. Radiology. 1981. 138:329–331.
25. el-Diasty TA, Shokeir AA, Tawfeek HA, Mahmoud NA, Nabeeh A, Ghoneim MA. Ethanol sclerotherapy for symptomatic simple renal cysts. J Endourol. 1995. 9:273–276.
26. Bozkurt FB, Boyvat F, Tekin I, Aytekin C, Coskun M, Ozkardes H. Percutaneous sclerotherapy of a giant benign renal cyst with alcohol. Eur J Radiol. 2001. 40:64–67.
27. Lee YR, Lee KB. Ablation of symptomatic cysts using absolute ethanol in 11 patients with autosomal-dominant polycystic kidney disease. Korean J Radiol. 2003. 4:239–242.
28. Suh DC, Shi HB, Park SS, Lee MS, Choi HY. Change of spontaneous reaction of glue and lipiodol mixture during embolization after the addition of tungsten powder: in vitro study. AJNR Am J Neuroradiol. 2000. 21:1277–1279.
29. Kim SH, Moon MW, Lee HJ, Sim JS, Kim SH, Ahn C. Renal cyst ablation with n-butyl cyanolate and iodized oil in symptomatic patients with autosomal dominant polycystic kidney disease: preliminary report. Radiology. 2003. 226:573–576.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download