Abstract
Objective
To evaluate the diagnostic accuracy of a dual-source computed tomography (DSCT) coronary angiography, with a particular focus on the effect of heart rate and calcifications.
Materials and Methods
One hundred and nine patients with suspected coronary disease were divided into 2 groups according to a mean heart rate (< 70 bpm and ≥ 70 bpm) and into 3 groups according to the mean Agatston calcium scores (≤ 100, 101-400, and > 400). Next, the effect of heart rate and calcification on the accuracy of coronary artery stenosis detection was analyzed by using an invasive coronary angiography as a reference standard. Coronary segments of less than 1.5 mm in diameter in an American Heart Association (AHA) 15-segment model were independently assessed.
Results
The mean heart rate during the scan was 71.8 bpm, whereas the mean Agatston score was 226.5. Of the 1,588 segments examined, 1,533 (97%) were assessable. A total of 17 patients had calcium scores above 400 Agatston U, whereas 50 had heart rates ≥ 70 bpm. Overall the sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV) for significant stenoses were: 95%, 91%, 65%, and 99% (by segment), respectively and 97%, 90%, 81%, and 91% (by artery), respectively (n = 475). Heart rate showed no significant impact on lesion detection; however, vessel calcification did show a significant impact on accuracy of assessment for coronary segments. The specificity, PPV and accuracy were 96%, 80%, and 96% (by segment), respectively for an Agatston score less than 100% and 99%, 96% and 98% (by artery). For an Agatston score of greater to or equal to 400 the specificity, PPV and accuracy were reduced to 79%, 55%, and 83% (by segment), respectively and to 79%, 69%, and 85% (by artery), respectively.
Coronary artery disease (CAD) is the major cause of morbidity and mortality in developed countries. An invasive coronary angiography (ICA) has been the gold standard method in diagnosing coronary stenosis lesions. Since 1998, research efforts have been devoted to develop reliable noninvasive electrocardiograph (ECG)-gated cardiac computed tomography (CT) examinations using multidetector CT (MDCT) systems in order to decrease the ICA-associated complications (1-3).
The rotation speed of the mechanical CT scanners has been a limiting factor in achieving sufficient temporal resolution. Since the development of the four-slice spiral CT scanners with rotation times of 500 msec (1, 2, 4-8) to the 64-slice spiral CT with rotation speeds of up to 330 msec and a temporal resolution of approximately 165 msec, coronary image quality has greatly improved; however, even for the new generation 64-slice spiral CT scanners, image quality is influenced by heart rate (9, 10). Therefore, beta-blockers have usually been prescribed as a premedication to achieve a heart rate below 60-65 bpm while scanning.
Recently, dual-source CT (DSCT) scanners have been introduced into clinics. DSCT combines two X-ray tube arrays and two corresponding detectors that are mounted onto a rotating gantry at a 90° angle, thus having a 0.4 mm isotropic resolution and a high temporal resolution of 83 msec, which is independent of heart rate in the monosegment reconstruction mode. Therefore, DSCT scanners can be expected to overcome image-degrading artifacts related to motion, pixel noise, or calcification.
A few studies demonstrated the possibilities with DSCT scanners with regards to visualizing the coronary arteries (11-14). However, to our knowledge, few studies which use DSCT scanners have investigated the diagnostic accuracy of a DSCT coronary angiography in parallel with an ICA or explored the effect of heart rate and calcification on the diagnostic accuracy. Therefore, the primary aim of this study was to assess the impact of heart rate and calcification on the diagnostic accuracy of DSCT scanning pertaining to significant coronary stenosis in relation to an ICA in 109 patients with suspected CAD.
Between November 2006 and November 2007, 109 patients (68 males, 41 females; mean age 63 ± 9 years; body mass index [BMI] ± standard deviation [SD], 26.9 ± 3.3 kg/m2) with suspected CAD were successively enrolled in this study and underwent both a DSCT coronary angiography and a conventional ICA within an interval of 1-30 days (mean ± SD, 10 ± 8 days). The exclusion criteria for DSCT were as follows: allergy to iodine-containing contrast medium, thyroid disorder, renal insufficiency (creatinine rates > 120 µmol/L), pregnancy, hemodynamic instability, and previous stent deployment or bypass surgery. Patients with high heart rates were included in the study. The patients were divided into two groups according to heart rates of < 70 bpm and ≥ 70 bpm or into three groups according to mean Agatston scores of < 100, 101-400, and > 400, respectively. The study protocol was approved by the local ethics committee (China) and all patients provided written informed consent.
All patients were scanned with a DSCT scanner (Somatom Definition, Siemens Medical Solutions, Germany). No beta-blockers were administered for the scan irrespective of the individual heart rate. The patients were placed in the supine position and positioned in the scanner so that the heart would be in the center of the scan field. Four ECG leads were attached to each patient's chest in the standard position. The ECG was continuously recorded and stored throughout the scan.
For calcium scoring, a non-enhanced DSCT was carried out from 1-1.5 cm below the level of the tracheal bifurcation to the diaphragm in the cranio-caudal direction and under the following conditions: tube potential 120 kV, tube current 100 mAs per rotation, detector collimation 32×0.6 mm, slice acquisition 64×0.6 mm by means of a z-flying focal spot, gantry rotation time of 330 msec, and pitch of 0.20-0.71 (adjusted to heart rate).
Next, a contrast-enhanced DSCT for a coronary angiography was performed and controlled by bolus tracking. The scan field is the same as described above for calcium scoring. After placing an 18-gauge catheter antecubitally for all patients, the DSCT scan was started by continuously injecting an iohexol bolus of 80 ml (iohexol, 350 mg iodine/ml, Amersham Health, Princeton, NJ) followed by 50 mL of a saline solution (injection rate 5 mL/s). A region of interest (ROI) was placed into the aortic root, and image acquisition began 5 s after the predetermined threshold of 80 Hounsfield units (HU) was attained. The work parameters for the DSCT were as follows: tube potential 120 kV, tube current 400 mAs per rotation, detector collimation beam of 32 × 0.6 mm, slice acquisition 64 × 0.6 mm using the z-flying focal spot technique, gantry rotation time of 330 msec, pitch of 0.2-0.5 (adjusted to the heart rate), and a field-of-view (FOV, mean 162 ± 15 mm; range 150-180 mm). The pitch was adjusted to the lowest expected heart rate while scanning.
A retrospective ECG-gated technique was applied to make the data reconstruction-synchronized. In the present study, a monosegment reconstruction algorithm was used for image reconstruction. The ECG pulsing window was 50-70% of the RR-interval with a step of 2%, which was first applied to reconstruct images for all patients. For patients with heart rate above 90 bpm, a window of 30-80% for the RR-interval was used. If necessary, additional images were reconstructed at 10-100% of the RR-interval in 10% increments. In the case of premature heart beats and irregular heart rates, the premature beating removing or arrhythmia shifting method was applied to improve the quality of image reconstruction (13, 15), respectively. The parameters used for image reconstruction for the DSCT coronary angiography, included a slice thickness of 0.75 mm, with increments of 0.5 mm and a kernel value B26f. The parameters used for image reconstruction for calcium scoring included a slice width of 3 mm and kernel of B35f. The DSCT data sets were transferred to an offline workstation (Wizard, Siemens Medical Solutions Software, Germany) with cardiac post-processing software (Syngo Circulation, Siemens Medical Solutions). Next to the axial images and oblique multiplanar reconstructions, curved multiplanar reconstructions (CMPR), maximum intensity projections (MIP), and three-dimensional volume rendering technique (VRT) reconstructions were created for visualization and analysis of the data. All data sets were independently analyzed by two observers and blinded to the patient's clinical data during the scan.
The ICA was performed according to the standard Judkins technique in all cases. To visualize the right coronary artery in at least two views, at least six views of the left coronary artery were performed, recorded, and stored. Coronary artery segments were classified according to the guidelines of the American Heart Association (AHA) (16). Stenosis severity was evaluated using a quantitative coronary analysis software (QCA, Innova 2000, GE Medical Systems, Waukesha, WI). A reduction in the minimal lumen diameter greater than 50% compared to the proximal reference was defined as significant stenosis. The angiograms were judged by one experienced cardiologist that was not involved in data read-out of the DSCT. All vessels with luminal diameters of at least 1.5 mm were analyzed.
Based on the Agatston score, coronary calcification was classified for each segment as mild (0-100 HU), moderate (101-400 HU), and heavy (≥ 400 HU) by a semi-automated software (Syngo Calcium Scoring, Siemens Medical Solutions).
The coronary arteries were segmented according to the same guidelines mentioned above (16). The right coronary artery was classified as segments 1-4, whereas the left main and left anterior descending artery as segments 5-10, and the left circumflex artery as segments 11-15. The intermedial artery was designated as segment 16, if present. All segments with a diameter ≥ 1.5 mm at their origin were included in the DSCT analysis.
All reconstructed images were independently evaluated by two experienced readers. The two readers were blinded to the ICA, and the related clinical information of the study subjects. All segments were blindly analyzed twice by each reader to evaluate the intra-observer reproducibility.
With the same criteria used for ICA analysis, significant stenosis was defined as greater than 50% narrowing of the coronary luminal diameter. Image quality was divided for each coronary segment into fine (no motion artifact, partial volume effect or pixel noise present to hamper image interpretation), adequate (some artifact or pixel noise present, but images acceptable and diagnostic), and poor (severe artifacts impairing accurate evaluation, non-assessable) as per Ehara et al. (17). The causes of image degradation were noted by both observers as arterial wall calcifications, motion artifacts, and others, such as low signal-to-noise ratio (SNR, SNR = CT value/SD), and disturbing adjacent structures. When the readings of the observers differed, a consensus was reached and used in the final analysis.
Quantitative variables were described as mean ± SD. Categorical variables were presented as counts and percentages. Inter-observer agreements were expressed as Cohen's kappa statistics. ICA was regarded as the reference standard. The diagnostic performance of the DSCT for the detection of significant stenosis was presented as sensitivity, specificity, PPV, NPV, and accuracy. Comparison between DSCT and QCA was made on a per-segment and per-artery level. The per-segment and per-artery accuracy in the subgroups of both heart rate and calcification was compared by the Chi-square test. Statistical analyses were performed with the SPSS software package (SPSS version 11.5, Chicago, IL). P < 0.05 was considered statistically significant (Figs. 1, 2).
Dual-source CT and ICA were both successfully performed in 109 patients without complications. The clinical characteristics of the enrolled patients are summarized in Table 1. The average heart rate during the scanning was 71.8 ± 13.2 bpm (range: 50-115 bpm). Fifty-nine patients (54%) had heart rates less than 70 bpm (mean: 58.6 ± 7.2 bpm, range: 50-69 bpm) and 50 patients (46%) had heart rates greater than 70 bpm (mean: 82.6 ± 9.1 bpm, range: 70-115 bpm).
The ICA revealed that the prevalence of significant CAD for the study population was 85 (78%), whereas four (4%) had a sole left main coronary artery lesion, 36 (33%) had 1-vessel CAD, 34 (31%) had 2-vessel CAD, and 11 (10%) had 3-vessel CAD. In total, 24 (22%) patients had no significant coronary artery stenosis.
The DSCT revealed that a significant proportion of patients had calcium deposits in their coronary vessels. Eighty-three patients (74%) had significant coronary artery stenosis, while 14 patients (13%) had calcifications without significant stenosis. The mean Agatston score for all the patients was 821 ± 904 (range 0-1,813). Fifty-five (51%) patients had an Agatston score between 0-100, 37 (34%) patients had an Agatston score between 101-400, and 17 (16%) patients had an Agatston score > 400.
In the present study, 39 segments were not shown by the DSCT due to variants, 77 segments had a diameter under 1.5 mm with 39 intermedial branches present. The image quality analysis by segment showed that the number of segments exhibiting a fine image quality was 1,273 (82%), adequate image quality was 260 (17%) and poor image quality was 25 (2%). In total, 1558 segments were imaged by ICA. Of these, 25 (2%) were not evaluated with a DSCT because of poor image quality. Thus, the study population was comprised of 1,533 segments for the evaluation of the accuracy of the DSCT for CAD detection. For an impaired image quality (including adequate and poor image quality), calcification (141 of 285, 50%) was the most common reason, followed by motion artifacts (101 of 285, 35%), low SNR (20 of 285, 7%), low contrast opacification (19 of 285, 7%), and overlaying adjacent structures (4 of 285, 1%). In patients with an Agatston score greater than 100, calcification accounted for decreased image quality 78% (110 of 141) of the time, while, a heart rate less than 70 bpm and motion artifacts accounted for 63% (64 of 101) of the impaired image quality.
The inter-observer kappa value for the DSCT evaluation of stenosis was 0.92, which indicates a good inter-observer agreement.
The CT dose index was about 30-42 mGy, which is similar to that by Flohr et al. (14). A total of 102 patients (94%) were successfully recognized by DSCT. Of the patients not recognized, two failed to show significant stenosis (false negative), and five patients were unsuccessfully recognized as having significant narrowing (false positive). A patient specific analysis revealed that the method sensitivity was 98% (83 of 85), specificity was 79% (19 of 24), PPV was 94% (81 of 86), NPV was 91% (21 of 23), and accuracy was 94% (102 of 109) (Table 2).
A total of 438 coronary vessels (92%) were correctly diagnosed using DSCT. Of the vessels that were not correctly diagnosed, five failed to show significant stenosis (false negative) and 32 vessels were incorrectly diagnosed as having significant narrowing (false positive). A per-artery analysis revealed that the overall method sensitivity was 97% (136 of 141), specificity was 90% (302 of 334), PPV was 81% (136 of 168), NPV was 98% (302 of 307), and accuracy was 92% (438 of 475) (Table 2).
A total of 1,408 (92%) segments were correctly diagnosed using DSCT. Of the segments incorrectly diagnosed, 12 segments failed to show significant stenosis (false negative) and 113 were incorrectly diagnosed as having significant narrowing (false positive). The causes associated with false positive and rating false negatives included massive calcification in 15 segments (13 false positive, 2 false negative) and motion artifacts in 9 segments (6 false positive, 3 false negative). A per-segment analysis revealed that overall sensitivity was 95% (213 of 225), specificity was 91% (1,195 of 1,308), PPV was 65% (213 of 326), NPV 99% (1,195 of 1,207), and accuracy was 92% (1,408 of 1,533) (Table 2).
In the subgroup of patients with a calcium rating, we examined the influence of calcium score on DSCT accuracy for the detection of stenosis in CAD patients. As seen in Table 3, the results demonstrated that when the calcium score was low (0 to 100 U), on a per-artery basis, sensitivity, specificity, PPV, NPV, and accuracy was 96% (47 of 49), 99% (140 of 142), 96% (47 of 49), 99% (140 of 142), and 98% (187 of 191), respectively. For a persegment basis, sensitivity, specificity, PPV, NPV, and accuracy was 91% (73 of 80), 96% (675 of 701), 74% (73 of 99), 99% (675 of 682), and 96% (748 of 781), respectively. DSCT remained highly accurate in the presence of moderate calcification, but when calcium score increased to 401 or higher, specificity, PPV, and accuracy was significantly reduced to 79% (72 of 91), 69% (43 of 62) and 85% (115 of 135) on a per-artery basis, and 79% (215 of 271), 55% (69 of 125) and 83% (284 of 342) on a per-segment basis, respectively.
In the heart-rate subgroups, we examined the influence of heart rate on DSCT accuracy for the detection of significant stenosis. The results showed a high degree of similarity for diagnostic accuracy of DSCT between the two heart rate subgroups (p < 0.05) (Table 4).
The present study demonstrated that DSCT scanning consistently provided a high diagnostic accuracy for CAD diagnosis, including patients with more elevated heart rates and coronary calcification. However, patients with heavy calcification (> 400 U) remain a challenge to diagnose.
Dual-source CT, with two arrays consisting of an X-ray tube, detectors arranged at a 90° angle, a gantry rotation time of 330 msec and a 32 × 0.6 mm collimation beam in combination with double z-sampling, allows for a temporal resolution of 83 msec independent of heart rate, submillimeter isotropic spatial resolution, and examination times no longer than 10 seconds (11, 14). The increased system parameters of DSCT can be expected to improve accuracy for CAD stenotic lesions. The results of our study showed that the diagnostic accuracy of DSCT was well improved compared to the prior DSCT. On a per-segment basis, the overall sensitivity, specificity, NPV and accuracy of DSCT of our results were 95%, 91%, 99%, and 92%, respectively. These results are consistent with Scheffel et al. (13) who cited a sensitivity of 96%, a specificity of 98%, a NPV of 99%, and an accuracy of 97% for a DSCT, respectively. However, the PPV (65% by segment) is lower than the 86% reported by Scheffel et al. (13). The improvement in image quality and diagnostic accuracy is determined by the characteristics of a DSCT. Our present report clearly demonstrated the advantages of a DSCT. The coronary arterial segments in patients with heart rates as 115 bpm could be visualized with greatly reduced motion artifact compared with earlier scanner generations (9, 10, 18-20). Only 2% of all segments had to be excluded from the analysis because of poor image quality. The DSCT showed that heart rate showed no significant influence on the diagnostic accuracy for CAD in the heart-rate subgroups, which is similar to a previous report (13).
In the present study, 54 (50%) of the study patients had calcified vessel walls. Recent studies have demonstrated vessel calcification was a serious limitation to the accurate assessment of coronary segments using a 64-slice CT (9, 21, 22). Raff et al. (9) reported a diagnostic accuracy in patients with Agatston scores above 400, markedly decreasing. The reported sensitivity, specificity, PPV and NPV was 93%, 67%, 93%, and 67%, respectively. Another recent study on calcification reported that the diagnostic accuracy of the 64-slice CT is degraded even in moderate calcification (calcium score ≥ 142) (21). The present results showed the efficacy of DSCT in ameliorating imaging difficulties with an overall sensitivity of 95%, specificity of 91%, PPV of 65% and NPV 99% on a per-segment basis, thus showing the increased diagnostic accuracy of DSCT except for the lower PPV. Our results are consistent with Scheffel et al. (13). Furthermore, the present study found that vessel calcification had a significant impact on the accurate assessment of coronary segments with a specificity of 96%, PPV of 80% and accuracy of 96% on a per-segment basis for the low calcium group (Agatston score < 100). In the high calcium group (Agatston score ≥ 400), the specificity, PPV and accuracy was reduced to 79%, 55%, and 83% respectively. On a per-artery basis, a specificity of 99%, PPV of 96% and accuracy of 98% was present in the low calcium and was reduced to 79%, 69%, and 85%, respectively in the high calcium group. Therefore, to some degree, a severe calcium burden remains an obstacle to diagnostic accuracy for CAD.
The present study examined a relatively large sample size (109 patients) to increase the validity and precision of our results compared to previous studies with DSCT for CAD detection (11-14). Furthermore, we included patients with unstable angina pectoris symptoms and our results suggested that DSCT might be used to differentiate coronary sources from other causes that may result in chest pain.
The present study was limited because it is a single-center research. Since all patients were referred to our centre for catheterization, there is a considerable patient selection bias in our series with a high prevalence of significant CAD (78%), which has a tendency to increase sensitivity. In the most calcified segments, the PPV was relatively poor (65%) and might even be lower in the general population with a far lower incidence of CAD. On the other hand, the high incidence of disease in these segments also influenced the NPV (99%). Further, patients with previous stent deployment or bypass surgery were not enrolled in the present study; therefore, further research is needed to establish the degree of accuracy in these settings.
In conclusion, the results of this study suggest that the DSCT coronary angiography provides a high overall diagnostic accuracy for the evaluation of CAD, even in patients with high heart rate and coronary calcification. Therefore, the use of the DSCT may have important clinical implications. A coronary DSCT could be a useful and noninvasive examination for the differentiation of acute chest pain and for the assessment of patients with suspected exercise test results. However, patients with severe calcification (> 400 U) remain a challenge to diagnose.
Figures and Tables
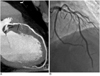
Fig. 1
Dual-source CT coronary angiography in 50-year-old man with suspected coronary artery disease (mean heart rate 88 bpm).
Curved-planar maximum-intensity projections (A) and three-dimensional volume rendering technique reconstructions (B) of left anterior descending artery both demonstrate significant artery stenosis of mid segment (arrow). Patient has one-vessel disease. Invasive coronary angiography (C) confirms significant stenosis of mid segment of right coronary artery (arrow).

Fig. 2
Dual-source CT coronary angiography in 50-year-old man with suspected coronary artery disease (Agatston score 823). Curved-planar maximum-intensity projections (A) of left anterior descending artery demonstrate significant stenosis of proximal and mid segment (arrow). However, invasive coronary angiography (B) in right anterior oblique cranial view shows mild to moderate degree of lumen reduction (< 50%) in proximal and mid segment (arrow) of left anterior descending artery, resulting in false positive diagnosis in dual-source CT coronary angiography. Patient has three-vessel disease and shows diffused calcification in left circumflex artery and right coronary artery (figure not shown).
Acknowledgement
We thank all the doctors at the CT center of the Shandong Medical Imaging Research Institute for their generous and valuable suggestions and help.
References
1. Ohnesorge B, Flohr T, Becker C, Kopp AF, Schoepf UJ, Baum U, et al. Cardiac imaging by means of electrocardiographically gated multisection spiral CT: initial experience. Radiology. 2000. 217:564–571.
2. Kachelriess M, Ulzheimer S, Kalender WA. ECG-correlated image reconstruction from subsecond multi-slice spiral CT scans of the heart. Med Phys. 2000. 27:1881–1902.
3. Taguchi K, Anno H. High temporal resolution for multislice helical computed tomography. Med Phys. 2000. 27:861–872.
4. Becker CR, Knez A, Leber A, Hong C, Treede H, Wildhirt S, et al. Initial experiences with multislice detector spiral CT in diagnosis of arteriosclerosis of coronary vessels. Radiologe. 2000. 40:118–122.
5. Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000. 102:2823–2828.
6. Nieman K, Oudkerk M, Rensing BJ, van Ooijen P, Munne A, van Geuns RJ, et al. Coronary angiography with multislice computed tomography. Lancet. 2001. 357:599–603.
7. Achenbach S, Giesler T, Ropers D, Ulzheimer S, Derlien H, Schulte C, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively ECG-gated, multislice spiral CT. Circulation. 2001. 103:2535–2538.
8. Knez A, Becker CR, Leber A, Ohnesorge B, Becker A, White C, et al. Usefulness of multislice spiral computed tomography angiography for determination of coronary artery stenoses. Am J Cardiol. 2001. 88:1191–1194.
9. Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005. 46:552–557.
10. Hoffmann MH, Shi H, Schmitz BL, Schmid FT, Lieberknecht M, Schulze R, et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005. 293:2471–2478.
11. Achenbach S, Ropers D, Kuettner A, Flohr T, Ohnesorge B, Bruder H, et al. Contrast-enhanced coronary artery visualization by dual-source computed tomography-initial experience. Eur J Radiol. 2006. 57:331–335.
12. Johnson TR, Nikolaou K, Wintersperger BJ, Leber AW, von Ziegler F, Rist C, et al. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006. 16:1409–1415.
13. Scheffel H, Alkadhi H, Plass A, Vachenauer R, Desbiolles L, Gaemperli O, et al. Accuracy of dual-source CT coronary angiography: first experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006. 16:2739–2747.
14. Flohr TG, McCollough CH, Bruder H, Petersilka M, Gruber K, Süss C, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006. 16:256–268.
15. Zhang Z, Jin Z, Zhang S, Lin S, Li D, Kong L, et al. Coronary artery imaging with dual-source CT: initial experience. Chin J Radiol. 2007. 41:973–976.
16. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975. 51:5–40.
17. Ehara M, Surmely JF, Kawai M, Katoh O, Matsubara T, Terashima M, et al. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: comparison with conventional invasive angiography. Circ J. 2006. 70:564–571.
18. Giesler T, Baum U, Ropers D, Ulzheimer S, Wenkel E, Mennicke M, et al. Noninvasive visualization of coronary arteries using contrast-enhanced multidetector CT: influence of heart rate on image quality and stenosis detection. AJR Am J Roentgenol. 2002. 179:911–916.
19. Schroeder S, Kopp AF, Kuettner A, Burgstahler C, Herdeg C, Heuschmid M, et al. Influence of heart rate on vessel visibility in noninvasive coronary angiography using new multislice computed tomography: experience in 94 patients. Clin Imaging. 2002. 26:106–111.
20. Hoffmann MH, Shi H, Manzke R, Schmid FT, De Vries L, Grass M, et al. Noninvasive coronary angiography with 16-detector row CT: effect of heart rate. Radiology. 2005. 234:86–97.
21. Ong TK, Chin SP, Liew CK, Chan WL, Seyfarth MT, Liew HB, et al. Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: influence of calcification. Am Heart J. 2006. 151:1323.
22. Brodoefel H, Reimann A, Burgstahler C, Schumacher F, Herberts T, Tsiflikas I, et al. Noninvasive coronary angiography using 64-slice spiral computed tomography in an unselected patient collective: effect of heart rate, heart rate variability and coronary calcifications on image quality and diagnostic accuracy. Eur J Radiol. 2008. 66:134–141.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download