Abstract
Gadobenate dimeglumine-enhanced magnetic resonance (MR) imaging simultaneously provides both morphological and functional information by the acquisition of dynamic and hepatobiliary-phase imaging. Focal lesions with no functioning hepatocytes, where hepatobiliary metabolism is blocked or inhibited, are generally unable to uptake and excrete gadobenate dimeglumine into the bile. Such lesions are typically malignant and usually appear hypointense as compared to the normal liver parenchyma as seen on hepatobiliary-phase imaging. However, various benign hepatic lesions may also be hypointense due to (a) the presence of no functioning hepatocytes, (b) damage to the functioning hepatocytes or (c) impairment of biliary function as depicted on hepatobiliary-phase imaging. All of these imaging features may result in recognition of the benign hepatic lesions as hepatic malignancies. As depicted on three-hour delayed hepatobiliary-phase imaging, peripheral iso/hyperintensity due to fibrotic tissue compared to the hypointense center with a fuzzy margin may be a clue for the presence of a benign hepatic lesion. In contrast, peripheral hypointensity due to rich tumoral cellularity compared to the center with a clear margin may favor an indication of the presence of a malignant hepatic lesion.
Gadobenate dimeglumine (gadolinium-benzyloxypropionictetraacetate [BOPTA], MultiHance; Bracco Imaging, Milan, Italy) can be used not only as a non-specific extracellular contrast agent for dynamic imaging of the liver, but also as a liver-specific agent for the acquisition of hepatobiliary-phase images (1-4). Therefore, gadobenate dimeglumine-enhanced magnetic resonance (MR) imaging simultaneously provides both morphological and functional information.
In general, lesions that contain functioning hepatocytes where hepatobiliary metabolism is mostly unaltered may be expected to uptake gadobenate dimeglumine in the same manner as normal hepatocytes and the lesions may be expected to excrete the compound into the bile. Such lesions are typically benign and usually appear isointense or hyperintense as compared to the normal liver parenchyma as depicted on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging. In contrast, lesions that do not contain functioning hepatocytes where hepatobiliary metabolism is blocked or inhibited are generally not able to uptake and excrete gadobenate dimeglumine into the bile. Such lesions are typically malignant and usually appear hypointense as compared to the normal liver parenchyma as depicted on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging (4-11). However, some benign lesions may be hypointense as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging and may be mistaken for hepatic malignancies, resulting in unnecessary performance of an interventional procedure such as a biopsy or surgery (4-13).
In this pictorial essay, we describe the mechanism of enhancement of gadobenate dimeglumine in the liver as seen on hepatobiliary-phase MR imaging. In addition, we will present various pathologically proven benign and malignant hypointense hepatic lesions as depicted on gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR imaging, with an emphasis on the differential diagnosis between benignancy and malignancy.
Gadobenate dimeglumine is a gadolinium-based contrast agent, which possesses a two-fold greater T1 relaxivity as compared to conventional gadolinium-based agents in human plasma, due to a weak and transient interaction with serum albumin (1, 2). Gadobenate dimeglumine undergoes elimination from the body through both a renal (95-97% of the injected dose) and hepatobiliary pathway (2, 3). Approximately 3-5% of the injected dose is taken up into the functioning hepatocytes through the action of organic anion transporting peptide 1 and the compound is eliminated into the bile by the adenosine triphosphate (ATP)-dependent canalicular multispecific organic anion transporter peptide (cMOAT), multidrug resistance-associated protein 2 (Mrp2) that transports bilirubin (Fig. 1) (14-18).
It is known that as seen on gadopentetate dimeglumine (Gd-DTPA)-enhanced 1-hour to 4-hour delayed MR imaging, the area with delayed enhancement corresponds pathologically to abundant fibrosis, the hypointense rim corresponds to rich tumoral cellularity and the hypointense center corresponds to coagulative necrosis (19). The enhancement of focal hepatic lesions as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging is affected by the internal component of the lesions such as the sinusoid-like vascular space or fibrosis, which is similar to the enhancement mechanism of Gd-DTPA. The enhancement of focal hepatic lesions is also affected by the retained hepatocyte activity of the lesion, which is unique as compared with the effect of Gd-DTPA (6, 19, 20). Appropriate gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging can be performed from 40 minutes after the injection of the contrast agent and the strong enhancement of normal liver parenchyma is maintained for a period of 120 minutes or longer (2, 18). In our institute, hepatobiliary-phase MR imaging has been obtained three hours after the injection of gadobenate dimeglumine for the minimization of the extracellular effects of the contrast agent.
A variety of benign hepatic lesions can appear hypointense as depicted on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging due to the following causes. (a) No functioning hepatocytes are present in the lesion, (b) damage to the functioning hepatocytes has occurred due to infection or inflammation and (c) impairment of biliary function in the lesion has occurred (4-13).
Cysts (Fig. 2) and hemangiomas (Fig. 3) are the most common benign lesions in the liver that lack functioning hepatocytes (5, 6). The differentiation of these lesions from other malignant hypointense hepatic lesions is possible with the use of unenhanced and gadobenate dimeglumine-enhanced dynamic MR imaging.
A variety of mesenchymal tumors of the liver with no functioning hepatocytes including angiomyolipomas and solitary fibrous tumors can appear as hypointense as depicted on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging; the appearance of these lesions may mimic a malignant tumor. We have experienced a rare mesenchymal tumor (21, 22), a hypointense solitary fibrous tumor with a clear margin (Fig. 4) as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging, which mimicked a malignant tumor.
We have experienced a reactive lymphoid hyperplasia (Fig. 5) in the liver that is a rare benign nodular lesion characterized by marked proliferation of non-neoplastic, polyclonal lymphocytes that form follicles with an active germinal center (23, 24). This nodule appeared hypointense with a clear margin as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging and mimicked a malignant tumor.
Focal eosinophilic liver lesions including focal eosinophilic infiltration, eosinophilic abscess and granuloma (Fig. 6) are composed of inflammatory cell infiltration with a large proportion of eosinophils with or without coagulation necrosis (25-27). Imaging findings of focal eosinophilic liver disease as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging depend on the degree of inflammation and the destruction of the liver parenchyma. The peripheral portion of an eosinophilic focal lesion may show retention of the contrast agent due to inflammation as compared with the central necrotic portion, which may result in the depiction of a fuzzy margin due to peripheral isointensity or hyperintensity relative to the center (Fig. 6).
With the use of gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging, imaging findings of inflammatory lesions (Fig. 7) such as hepatic abscesses may vary depending on the difference in the degree of inflammation and destroyed hepatic parenchyma (11, 13). The presence of peripheral isointensity or hyperintensity of an inflammatory lesion may be mainly due to the retention of the contrast agent in fibrous tissues, from an inflammatory change due to extensive fibrosis as relative to the hypointensity (necrosis) of the center, and may suggest that the lesion is not typically malignant in nature.
Although a hepatic adenoma (Fig. 8) has functioning hepatocytes, it lacks bile ducts (7, 8, 28). Therefore, it is likely that the hepatocellular bilirubin metabolism is blocked in the adenoma, as confirmed by the absence of bile in a resected adenoma (28), which results in the absence of uptake and transport of gadobenate dimeglumine. As seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging, an adenoma appears as a hypointense lesion. Although not always helpful, for the successful differential diagnosis of hepatic adenomas from other malignant hypointense hepatic lesions, especially hepatocellular carcinomas, the use of unenhanced and gadobenate dimeglumine-enhanced dynamic MR imaging may be complementary.
Dysplastic nodules usually appear isointense as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging (9, 10). The biliary function of a low-grade dysplastic nodule is usually preserved and bile ducts are present in portal areas. In a high-grade dysplastic nodule, the biliary function may only be partially impaired, in which portal triads may be focally absent in the nodule and thus bile ducts may be missing (4). Although confirmation of the presence of the tumor after performing a needle biopsy is not always sufficient to eliminate the need for a histological examination of the entire tumor after resection (Fig. 9), uptake and transport of gadobenate dimeglumine may be impaired in a dysplastic nodule.
Malignant lesions such as advanced hepatocellular carcinomas (Fig. 10), cholangiocarcinomas (Fig. 11) and metastases (Fig. 12) do not contain functioning hepatocytes and thus cannot retain gadobenate dimeglumine. These lesions appear hypointense as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging (4-6, 9, 30).
Malignant hepatic lesions tend to outgrow their blood supply, producing central necrosis, hemorrhage and fibrosis, which results in the depiction of various imaging patterns on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging. A target appearance that is defined as peripheral hypointensity relative to the center (6, 20) may appear in a malignant mass with abundant fibrosis such as a cholangiocarcinoma and a metastasis. The pathological features may explain the target appearance, where the majority of stromal fibrosis is in the center of the lesions, resulting in the retention of gadobenate dimeglumine in the center. More abundant tumoral cellularity is observed in the periphery of the lesion, resulting in rim-like hypointensity to the center, which may be similar to the enhancement mechanism of the target appearance by Gd-DTPA (29, 30). The target appearance appears to be more conspicuous on Gd-DTPA-enhanced hepatobiliary-phase images than Gd-DTPA-enhanced delayed images due to the marked and prolonged enhancement of the normal liver parenchyma as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase images.
In conclusion, gadobenate dimeglumine has a property of being a unique liver-specific contrast agent in addition to a property of being an extracellular contrast agent, which results in the marked and prolonged enhancement of the normal liver parenchyma up to three hours after the administration of the contrast agent with intralesional enhancement. Based on these features, three-hour delayed hepatobiliary-phase MR imaging may be helpful in the characterization of focal liver lesions. Both benign and malignant hepatic lesions may appear hypointense as seen on gadobenate dimeglumine-enhanced hepatobiliary-phase MR imaging. Benign hypointense hepatic lesions as seen on hepatobiliary-phase imaging alone may be mistaken for hepatic malignancies, resulting in the performance of an unnecessary biopsy or surgery. As seen on three-hour delayed hepatobiliary-phase imaging, peripheral iso/hyperintensity due to fibrotic tissue compared to the hypointense center with a fuzzy margin may be a clue of the presence of a benign hepatic lesion. In contrast, peripheral hypointensity due to rich tumoral cellularity compared to the center with a clear margin may favor the presence of a malignant hepatic lesion.
Figures and Tables
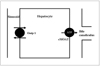 | Fig. 1Schematic illustration of proposed sinusoidal and canalicular transport pathway of gadobenate dimeglumine in rat liver. Approximately 3-5% of injected dose is taken up into functioning hepatocytes by organic anion transporting polypeptide1 (Oatp1) and is eliminated into bile by action of adenosine triphosphate (ATP)-dependent canalicular multispecific organic anion transporter peptide (cMOAT), multidrug resistance-associated protein 2 that transports bilirubin. |
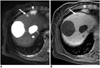 | Fig. 263-year-old woman with hepatic cysts and hepatocellular carcinoma that were confirmed after hepatic resection.
A. Respiratory-triggered single-shot T2-weighted MR image shows typical hyperintense cysts. Small hyperintense lesion (arrow) is cyst. Enlargement of lymph node (arrowhead) in right cardiophrenic area is noted.
B. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows multiple hypointense lesions. Small hypointense lesion (arrow) is cyst. Enlargement of lymph node (arrowhead) in right cardiophrenic area is noted.
|
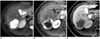 | Fig. 332-year-old woman with hemangioma that was confirmed by typical CT and MR imaging findings.
A. Respiratory-triggered single-shot T2-weighted MR image shows very hyperintense lesion in right liver.
B. Gadobenate dimeglumine-enhanced arterial-phase MR image shows typical peripheral globular enhancement of hemangioma.
C. Transverse three-hour delayed hepatobiliary-phase MR image shows discrete hypointense lesion.
|
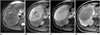 | Fig. 447-year-old woman with solitary fibrous tumor that was confirmed by hepatic resection.
A. Respiratory-triggered single-shot T2-weighted MR image shows heterogeneous hypointense and hyperintense mass with central hyperintense area.
B, C. Gadobenate dimeglumine-enhanced arterial- (B) and equilibrium-phase (C) MR images show heterogeneous arterial hypervascular enhancing mass, followed by persistent homogeneous enhancement except for central area.
D. Three-hour delayed hepatobiliary-phase MR image shows discrete hypointense mass with enhancement of central area due to fibrosis.
|
 | Fig. 561-year-old woman with reactive lymphoid hyperplasia that was confirmed after hepatic resection in liver. Patient had colon cancer.
A. Contrast-enhanced CT scan obtained during portal-phase shows discrete hypodense nodule (arrow) that mimicked metastatic nodule in left liver.
B. Respiratory-triggered single-shot T2-weighted MR image shows hyperintense nodule (arrow).
C. Gadobenate-dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows hypointense nodule with clear margin (arrow) that mimicked metastatic nodule in left liver.
|
 | Fig. 668-year-old woman with eosinophilic granuloma that was confirmed after hepatic resection. Patient had history of surgery for colon cancer two years prior.
A. Contrast-enhanced CT scan obtained during portal-phase shows hypodense nodule (arrow) with peripheral ring-like enhancement in left liver.
B. Respiratory-triggered single-shot T2-weighted MR image obtained 12 days after CT examination shows hyperintense nodule (arrow).
C. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image obtained 12 days after CT examination shows lesion (arrow) with peripheral isointensity and central hypointensity.
D. Photomicrograph of resected specimen shows inflammatory cell infiltration with fibroblast proliferation (asterisks), which appeared to be ring enhancement as seen on hepatobiliary-phase MR image. Necrosis (N) corresponds to central hypointensity as seen on the hepatobiliary-phase MR image. L = normal liver (Hematoxylin & Eosin staining, ×100).
|
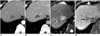 | Fig. 745-year-old man with inflammatory mass that was confirmed after percutaneous biopsy in normal liver.
A, B. Contrast-enhanced CT scans obtained during arterial- (A) and equilibrium-phase (B) show discrete hypodense mass with peripheral rim-like enhancement in right liver that mimicked cholangiocarcinoma.
C. Respiratory-triggered single-shot T2-weighted MR image obtained three weeks after CT examination shows hyperintense mass.
D. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image obtained three weeks after CT examination shows mass (arrow) with peripheral isointensity compared to hypointense center. This lesion showed decrease in size nine months after initial CT examination.
|
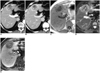 | Fig. 873-year-old man with hepatic adenoma that was confirmed after hepatic resection.
A, B. Contrast-enhanced CT scans obtained during arterial- (A) and equilibrium-phase (B) show mass with peripheral hyperenhancement, followed by washout with peripheral rim-like enhancement and central hypodensity due to hemorrhage.
C, D. T1-weighted (C) and respiratory-triggered single-shot T2-weighted (D) MR images show mass with slight hypointensity and hyperintensity, respectively, which may be differential characterization features from malignant tumor such as advanced hepatocellular carcinoma. Central hemorrhage is noted.
E. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows discrete heterogeneous hypointense mass.
|
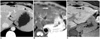 | Fig. 944-year-old woman with low-grade dysplastic nodule that was confirmed after percutaneous biopsy in normal liver. Patient did not have viral infection.
A. Contrast-enhanced CT scan obtained during equilibrium-phase shows small hypodense nodule (arrow) with arterial hypovascularity (data not shown) in left liver. Two similar hypodense nodules (arrowheads) are noted in left liver.
B. Respiratory-triggered single-shot T2-weighted MR image shows hypointense nodule (arrow).
C. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows discrete hypointense nodule (arrow). This nodule and another two nodules in left liver showed no change in size 16 months after initial CT examination.
|
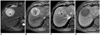 | Fig. 1045-year-old man with hepatocellular carcinoma with central scar that was confirmed after hepatic resection.
A. Respiratory-triggered single-shot T2-weighted MR image shows hyperintense mass in right liver. Central scar is very bright (arrowhead).
B, C. Gadobenate dimeglumine-enhanced MR images obtained during arterial- (B) and equilibrium-phase (C) show typical enhancement (hypervascular with washout pattern) of hepatocellular carcinoma with delayed central hyperenhancement due to presence of fibrous scar (arrowheads).
D. Three-hour delayed hepatobiliary phase MR image shows discrete homogeneous hypointense mass.
|
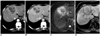 | Fig. 1161-year-old woman with cholangiocarcinoma that was confirmed after percutaneous biopsy.
A, B. Contrast-enhanced CT scans obtained during arterial- (A) and equilibrium- (B) show irregular and peripheral enhancing mass, followed by progressive heterogeneous central enhancement seen in liver.
C. Respiratory-triggered single-shot T2-weighted MR image shows peripheral hyperintense mass with central hypointensity due to fibrosis.
D. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows mass with peripheral hypointensity compared to center.
|
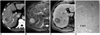 | Fig. 1268-year-old man with hepatic metastasis that was confirmed after hepatic resection. Patient had sigmoid colon cancer.
A. Contrast-enhanced CT scan obtained during portal-phase shows mass with peripheral rim-like enhancement in right liver.
B. Respiratory-triggered single-shot T2-weighted MR image shows hyperintense mass.
C. Gadobenate dimeglumine-enhanced three-hour delayed hepatobiliary-phase MR image shows mass with peripheral hypointensity compared to center.
D. Photomicrograph of resected specimen shows rich tumoral cellularity (asterisks) in periphery of metastatic nodule, which is seen with peripheral hypointensity compared to center that consisted of fibrosis and necrosis (F & N) as depicted on hepatobiliary-phase MR image. L = normal liver (Hematoxylin & Eosin staining, ×50).
|
References
1. Cavagna FM, Maggioni F, Castelli PM, Daprà M, Imperatori LG, Lorusso V, et al. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997. 32:780–796.
2. Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998. 33:798–809.
3. Spinazzi A, Lorusso V, Pirovano G, Kirchin M. Safety, tolerance, biodistribution and MR imaging enhancement of the liver with gadobenate dimeglumine: results of clinical pharmacologic and pilot imaging studies in nonpatient and patient volunteers. Acad Radiol. 1999. 6:282–291.
4. Bartolozzi C, Crocetti L, Lencioni R, Cioni D, Della Pina C, Campani D. Biliary and reticuloendothelial impairment in hepatocarcinogenesis: the diagnostic role of tissue-specific MR contrast media. Eur Radiol. 2007. 17:2519–2530.
5. Petersein J, Spinazzi A, Giovagnoni A, Soyer P, Terrier F, Lencioni R, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging-a multicenter phase III clinical study. Radiology. 2000. 215:727–736.
6. Kim YK, Lee JM, Kim CS. Gadobenate dimeglumine-enhanced liver MR imaging: value of dynamic and delayed imaging for the characterization and detection of focal liver lesions. Eur Radiol. 2004. 14:5–13.
7. Grazioli L, Morana G, Federle MP, Brancatelli G, Testoni M, Kirchin MA, et al. Focal nodular hyperplasia: morphologic and functional information from MR imaging with gadobenate dimeglumine. Radiology. 2001. 221:731–739.
8. Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology. 2005. 236:166–177.
9. Leen E. MultiHance-enhanced MRI in the characterisation of focal liver lesions. Eur Radiol. 2004. 14:O31–O35.
10. Kim JI, Lee JM, Choi JY, Kim YK, Kim SH, Lee JY, et al. The value of gadobenate dimeglumine-enhanced delayed phase MR imaging for characterization of hepatocellular nodules in the cirrhotic liver. Invest Radiol. 2008. 43:202–210.
11. Runge VM, Wells JW, Williams NM. Hepatic abscess. Magnetic resonance imaging findings using gadolinium-BOPTA. Invest Radiol. 1996. 31:781–788.
12. Marin D, Iannaccone R, Catalano C, Passariello R. Multinodular focal fatty infiltration of the liver: atypical imaging findings on delayed T1-weighted Gd-BOPTA-enhanced liver-specific MR images. J Magn Reson Imaging. 2006. 24:690–694.
13. Schneider G, Fries P, Samaras P, Remberger K, Uder M, Kramann B. Inflammatory pseudotumor of the liver in a patient with congenital granulocytopenia and HCV infection. Eur J Radiol. 2003. 48:293–298.
14. Pascolo L, Petrovic S, Cupelli F, Bruschi CV, Anelli PL, Lorusso V, et al. Abc protein transport of MRI contrast agents in canalicular rat liver plasma vesicles and yeast vacuoles. Biochem Biophys Res Commun. 2001. 282:60–66.
15. Pastor CM, Planchamp C, Pochon S, Lorusso V, Montet X, Mayer J, et al. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology. 2003. 229:119–125.
16. Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997. 26:1667–1677.
17. Stanca C, Jung D, Meier PJ, Kullak-Ublick GA. Hepatocellular transport proteins and their role in liver disease. World J Gastroenterol. 2001. 7:157–169.
18. Spinazzi A, Lorusso V, Pirovano G, Taroni P, Kirchin MA, Davies A. Multihance clinical pharmacology: biodistribution and MR enhancement of the liver. Acad Radiol. 1998. 5:S86–S89.
19. Gabata T, Matsui O, Kadoya M, Yoshikawa J, Ueda K, Kawamori Y, et al. Delayed MR imaging of the liver: correlation of delayed enhancement of hepatic tumors and pathologic appearance. Abdom Imaging. 1998. 23:309–313.
20. Mahfouz AE, Hamm B, Wolf KJ. Peripheral washout: a sign of malignancy on dynamic gadolinium-enhanced MR images of focal liver lesions. Radiology. 1994. 190:49–52.
21. Nath DS, Rutzick AD, Sielaff TD. Solitary fibrous tumor of the liver. AJR Am J Roentgenol. 2006. 187:W187–W190.
22. Fuksbrumer MS, Klimstra D, Panicek DM. Solitary fibrous tumor of the liver: imaging findings. AJR Am J Roentgenol. 2000. 175:1683–1687.
23. Machida T, Takahashi T, Itoh T, Hirayama M, Morita T, Horita S. Reactive lymphoid hyperplasia of the liver: a case report and review of literature. World J Gastroenterol. 2007. 13:5403–5407.
24. Takahashi H, Sawai H, Matsuo Y, Funahashi H, Satoh M, Okada Y, et al. Reactive lymphoid hyperplasia of the liver in a patient with colon cancer: report of two cases. BMC Gastroenterol. 2006. 6:25.
25. Fauci AS, Harley JB, Roberts WC, Ferrans VJ, Gralnick HR, Bjornson BH. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982. 97:78–92.
26. Lee WJ, Lim HK, Lim JH, Kim SH, Choi SH, Lee SJ. Foci of eosinophil-related necrosis in the liver: imaging findings and correlation with eosinophilia. AJR Am J Roentgenol. 1999. 172:1255–1261.
27. Lim JH, Lee KS. Eosinophilic infiltration in Korea: idiopathic? Korean J Radiol. 2006. 7:4–6.
28. Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988. 208:558–564.
29. Caudana R, Morana G, Pirovano GP, Nicoli N, Portuese A, Spinazzi A, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA)-preliminary results of phase II clinical application. Radiology. 1996. 199:513–520.
30. Muramatsu Y, Takayasu K, Moriyama N, Shima Y, Goto H, Ushio K, et al. Peripheral low-density area of hepatic tumors: CT-pathologic correlation. Radiology. 1986. 160:49–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download