Abbreviations
ALSA
ARSA
CAA
IAA
LAA
LSA
MDCT
MinIP
MIP
MPR
RAA
VR
Journal List > Korean J Radiol > v.10(2) > 1026318
ALSA
ARSA
CAA
IAA
LAA
LSA
MDCT
MinIP
MIP
MPR
RAA
VR
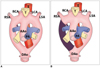 | Fig. 1Diagram of Edward's developmental model of aortic arch. A. According to hypothetical double aortic arch system in which there is aortic arch and ductus arteriosus on each side, carotid and subclavian arteries arise from their respective arches. Descending aorta is in midline. B. Normal branching arch results from interruption of dorsal segment of right arch between right subclavian artery and descending aorta, with regression of right ductus arteriosus. AAo = ascending aorta, DAo = descending aorta, PA = pulmonary artery, T = trachea, E = esophagus, RSA = right subclavian artery, RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery |
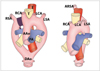 | Fig. 2Diagram of development of left aortic arch with aberrant right subclavian artery is presented. This anomaly results from interruption of dorsal segment of right arch between right carotid artery and right subclavian arteries with regression of right ductus arteriosus in hypothetical double aortic arch. AAo = ascending aorta, DAo = descending aorta, PA = pulmonary artery, T = trachea, E = esophagus, RSA = right subclavian artery, RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery, ARSA = aberrant right subclavian artery |
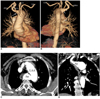 | Fig. 3Left aortic arch (LAA) with aberrant right subclavian artery (ARSA) in 66-year-old woman with dysphagia is shown. Anterior (A) and posterior (B) volume rendering images show ARSA with Kommerell's diverticulum at its origin (white arrows). Right carotid artery (RCA) arises as first branch from aortic arch, which is followed by left carotid artery (LCA), left subclavian artery (LSA) and ARSA. Axial (C) and coronal multiplanar reformatted (D) images show presence of aneurysm (An) of Kommerell's diverticulum with mural thrombus (T) and calcifications. There is compression of esophagus (black arrow). |
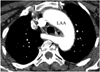 | Fig. 4Left aortic arch (LAA) with aberrant right subclavian artery in 48-year-old woman with anomalous origin of right vertebral artery from right common carotid artery is shown. Axial image shows retroesophageal course of aberrant right subclavian artery (arrow). |
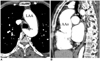 | Fig. 5Aberrant right subclavian artery in 53-year-old woman with severe aortic coarctation presenting with hypertension and dysphagia. Axial (A) and sagittal multiplanar reformatted (B) images show left aortic arch (LAA) with aberrant right subclavian artery (long arrows) causing esophageal compression (short arrows). Paravertebral collaterals and prominent internal mammarian arteries are also seen. AAo = ascending aorta |
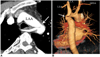 | Fig. 6Aberrant right subclavian artery in 48-year-old man with aortic coarctation presenting with hypertension. Axial oblique (A) and posterior volume rendering (B) images show aberrant right subclavian artery (ARSA) arising just distal to aortic coarctation (arrows). LAA = left aortic arch, LSA = left subclavian artery, LCA = left carotid artery, RCA = right carotid artery |
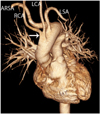 | Fig. 7Aberrant right subclavian artery in 51-year-old woman with dysphagia is shown. Anterior volume rendering image shows aberrant right subclavian artery (ARSA) and common origin of carotid arteries (arrow). RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery |
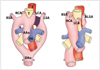 | Fig. 8Diagram of development of right aortic arch with aberrant left subclavian artery is shown. Anomaly results from interruption of dorsal segment of left arch between left common carotid artery and left subclavian arteries with regression of right ductus arteriosus in hypothetical double aortic arch. AAo = ascending aorta, DAo = descending aorta, PA = pulmonary artery, T = trachea, E = esophagus, RSA = right subclavian artery, RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery, ALSA = aberrant left subclavian artery |
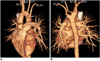 | Fig. 9Right aortic arch with aberrant left subclavian artery in 45-year-old asymptomatic woman is shown. Anterior (A) and posterior (B) volume rendering images show right aortic arch (RAA) with aberrant left subclavian artery (ALSA) associated with Kommerell's diverticulum (arrows) at its origin. First branch arising from aortic arch is left carotid artery (LCA), which is followed by right carotid artery (RCA), right subclavian artery (RSA) and ALSA. |
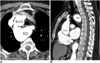 | Fig. 10Right aortic arch (RAA) with aberrant left subclavian artery in 63-year-old woman without dysphagia. Axial (A) and sagittal multiplanar reformatted (B) images show aneurysmal Kommerell's diverticulum (KD) with 3.2 cm diameter causing esophageal compression (arrows). AAo = ascending aorta |
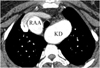 | Fig. 11Right aortic arch (RAA) with aberrant left subclavian artery in 52-year-old-woman with dysphagia is presented. Axial image shows aneurysmal Kommerell's diverticulum (KD) with 4 cm diameter. |
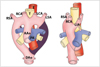 | Fig. 12Diagram of development of right aortic arch with mirror image branching. Anomaly results from interruption of dorsal segment of left arch between left subclavian artery and descending aorta with regression of right ductus arteriosus in hypothetical double aortic arch. AAo = ascending aorta, DAo = descending aorta, PA = pulmonary artery, T = trachea, E = esophagus, RSA = right subclavian artery, RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery, LIA = left innominate artery |
 | Fig. 13Right aortic arch with mirror image branching in 20-year-old woman with tetralogy of Fallot, aortopulmonary collateral arteries, partial anomalous pulmonary venous connection, double superior vena cava and double inferior vena cava with azygos continuation. Coronal volume rendering (A) and axial (B, C) images show left innominate artery (LIA) is first branch arising from arch, which is followed by right carotid artery (RCA) and right subclavian artery (RSA). Prominent left internal mammarian artery, mediastinal collateral vessels and dilatation of ascending and arcus aorta are also seen. AAo = ascending aorta, LCA = left carotid artery, LSA = left subclavian artery, RAA = right aortic arch |
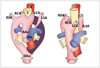 | Fig. 14Diagram of development of right aortic arch with isolated left subclavian artery is shown. Anomaly results from interruption of left arch at two levels; one level is between left common carotid and left subclavian arteries and other level is distal to attachment of left ductus. Left subclavian artery does not have connection with aorta, but is connected to pulmonary artery by left ductus arteriosus. AAo = ascending aorta, DAo = descending aorta, PA = pulmonary artery, T = trachea, E = esophagus, RSA = right subclavian artery, RCA = right carotid artery, LCA = left carotid artery, LSA = left subclavian artery, ILSA = isolated left subclavian artery |
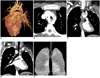 | Fig. 15Double aortic arch in 31-year-old woman presenting with dysphagia is shown. Volume rendering (A) image shows that right arch is larger and extends more cephalad than left arch. Brachiocephalic arteries arise from their respective arches. Axial maximum intensity projection images (B), coronal multiplanar reformatted images (C, D) and volume rendering image with lower opacity values (E) show presence of complete vascular ring causing esophageal (arrows) and tracheal (T) compression. AAo = ascending aorta, DAo = descending aorta, R = right arch, L = left arch, RCA = right carotid artery, RSA = right subclavian artery, LCA = left carotid artery, LSA = left subclavian artery |
ALSA
ARSA
CAA
IAA
LAA
LSA
MDCT
MinIP
MIP
MPR
RAA
VR