Abstract
Objective
To evaluate the feasibility of CT fluoroscopy (CTF)-guided percutaneous transhepatic biliary drainage (PTBD) in emergency patients with acute obstructive cholangitis.
Materials and Methods
The study included 28 patients admitted to the emergency center due to obstructive jaundice and found to require urgent biliary drainage, as well as judged to have a suitable peripheral bile duct for a CTF-guided puncture (at least 4 mm in width). Prior to the CTF-guided puncture, a CT scan was performed to evaluate bile duct dilatation and the underlying causes of biliary obstruction. If the patient was judged to be a suitable candidate, a CTF-guided PTBD was performed in the same CT unit without additional fluoroscopic guidance. Technical feasibility of the procedure was investigated with the evaluation of overall success rate and causes of failure.
Results
A hepatic puncture was attempted at the left lobe in 23 patients and right lobe in five patients. The procedure was successful in 24 of 28 patients (86%) Successful biliary puncture was achieved on the first attempt in 16 patients, the second attempt in five patients, and the third attempt in three patients. The causes of failure included guide wire twisting in one patient, biliary puncture failure in two patients, and poor visualization of the guide wire in one patient. There were no significant procedure-related complication.
Conclusion
The CTF-guided PTBD is technically feasible and highly successful in patients judged to have a suitable indication. Moreover, although the procedure is unfamiliar and inconvenient to interventionalists, it has economical advantages in that it saves time and manpower. We believe this method can be used in the emergency patients requiring urgent biliary drainage as an alternative for the fluoroscopy-guided PTBD.
Since the first clinical introduction by Katada et al. (1, 2) in 1994, real time CT fluoroscopy (CTF) has been increasingly used in the field of interventional radiology. Previous reports have described advantages of CTF in the biopsy, drainage, and percutaneous placement of needles or other interventional instruments into the target locations (3-10). CTF had also previously been applied to percutaneous transhepatic biliary drainage (PTBD) and the results were already reported (11-13). However, these results were not purely for CTF-guided PTBD, but for PTBD using the combined guidance of CTF and mobile C-arm fluoroscopy. CTF was used only for the puncture of suitable peripheral bile ducts, and the rest of work was completed under the guidance of C-arm fluoroscopy. Generally, patients with obstructive jaundice require two steps to undergo palliative PTBD. A CT scan or sonography is first performed to evaluate the degree of biliary dilatation and its underlying causes. Next, the patients are moved to intervention unit for the procedure. We believe that these steps could be simplified using CTF, which would be beneficial for the patients and clinical staff. The purpose of this study is to evaluate the clinical feasibility of CTF-guided PTBD as a means of a simplification in the emergency work steps of patients requiring urgent biliary drainage.
We performed CTF-guided PTBD on five cases as part of a pilot trial before the main study to familiarize ourselves with the procedure. The main study had been prospectively performed for a total of 11 months after the pilot trial. The study subjects that were patients hospitalized at the emergency center due to acute cholangitis by biliary obstruction. They clinically complained of fever, abdominal pain at the right upper quadrant, and jaundice. The laboratory tests performed on them revealed elevated serum bilirubin levels and leukocytosis. A CT scan (Somatom plus 4, Siemens Medical Systems, Erlangen, Germany) was performed to evaluate bile duct dilatation and underlying causes. Among them, we selected 28 patients (12 M:16 F, age range 48-82 yrs old, mean 62 yrs old) according to following inclusion criteria; 1) Urgent biliary drainage was clinically required. 2) Taking into account a rough resolution of the real-time CTF image, the width of the peripheral bile duct should be at least 4 mm or more for the CTF-guided puncture. 3) Patients and their families had to have sufficient understanding of the procedure to allow a CTF-guided PTBD to be performed. Following the diagnostic CT scanning, selected patients underwent CTF-guided PTBD in the CT room without moving to the intervention unit. Using the C.A.R.E vision system (Siemens Medical Systems, Erlangen, Germany), real-time CTF was acquired in continuous mode (80-90 kVp, 75 mAs, 6 frames per sec, 8 mm thickness). The details of the procedure were as follows; 1) evaluation of the anatomy of the dilated bile ducts and the underlying causative lesions on conventional contrast enhancement CT, 2) determination of the suitable puncture location for the bile duct and the imaginary tracing of the bile duct through which a guide wire and catheter will pass. 3) sterile preparation for the skin, draping, and local anesthesia at the puncture site with 2% lidocaine, 4) percutaneous bile duct puncture with an 18 or 21 G needle under realtime monitoring with CTF, 5) using CTF as a monitoring method, instrument insertion through bile duct; guide wire (0.018" wire in 21 G needle / 0.035" wire in 18 G needle), dilator, and 8 Fr drainage catheter in order, 6) after placing a drainage catheter in the appropriate location (common bile duct [CBD] in the distal obstruction and hilar duct in the proximal obstruction), contrast media infusion through the catheter to facilitate performing a CT topography. The success rate, number of needling for successful bile duct puncture, and technical limitations of the procedure were evaluated with measurement of time spent for the procedure.
The cause of biliary obstruction was CBD stone in 16 patients, distal CBD cancer in eight patients, and hilar cholangiocarcinoma in four patients. Bile duct puncture was attempted at the left lobe in 23 patients and at the right lobe in five patients. CTF-guided PTBD was successful in 24 of 28 patients (86%) (Fig. 1). Successful bile duct puncture was achieved on the first trial in 16 patients, second trial in five patients and third trial in three patients. The causes of failure included bile duct puncture failure in two patients, guide wire (0.018") twisting in one patient, and poor visualization of the guide wire due to pre-injected contrast media in one patient (Fig. 2). All of them occurred in the left lobe approach. Failure of the bile duct puncture was due to poor respiratory control of the patients. Twisting of the guide wire occurred during its advancement through the bile duct. In this case, a guide wire was lodged somewhere in the bile duct and could no longer be manipulated. The four patients which failed to successfully undergo the complete procedure were moved to the intervention unit and PTBD was successfully completed in three patients. One patient also failed even in the intervention unit due to poor respiratory control. The total elapsed time from bile duct puncture to drainage catheter insertion was 5 to 12 minutes in successful cases. There were no significant procedure-related complications in all patients.
CT fluoroscopy is a recently introduced imaging tool, which facilitates the acquisition of a real-time monitoring of sectional CT images (1, 2). It has been reported that CTF has clinical advantages with regards to biopsy, fluid drainage, local placement of needle for drug injection or other interventional procedures, and radio-frequency ablation (3-10). In spite of the disputes corresponding to differences in opinion regarding the radiation dose (14, 15), the clinical application of CTF has further been extended (16-20). According to previous studies, CTF has the potential to deliver a higher radiation dose and exposure, which can be compensated for by adopting the lowest current, using lead drapes on the patients or needle holder, and a decrease of procedure time by quick needle targeting (5, 21).
To our knowledge, a CTF-guided PTBD has been attempted in only three published studies (11-13). Patients with malignant biliary obstructions were enrolled in the studies, which involved a planned stent-assisted recanalization following a temporary PTBD. Therefore, the purpose of CTF guiding was the puncturing of suitable peripheral duct that had an obtuse angle with the central bile duct. After puncturing the bile duct, the rest of the procedures were performed under the guidance of mobile C-arm fluoroscopy. The studies concluded that CTF guiding had its advantages, which involved the visualization of the most suitable bile duct for puncture and reduced time and number of puncture trial as compared with conventional fluoroscopy guided PTBD.
We attempted CTF guided PTBD in the patients who needed urgent biliary drainage. All procedures, from the bile duct puncture to the drainage catheter placement, were performed under CTF guidance without C-arm fluoroscopy. The purpose of the trial was to evaluate the technical feasibility of CTF-guided PTBD and the possibility of one step PTBD in the CT unit immediately after diagnostic CT scanning without moving patients into the intervention unit. The procedure was technically feasible for most patients, however a little modification was needed in the technique based on what was described in previous reports (11-13). A right lobe puncture was preferred in previous studies to ensure the appropriate tracts for stent delivery. It was a reasonable decision and did not result in problems in terms of PTBD, because the next steps were performed under C-arm fluoroscopy guidance. However, one step CTF-guided PTBD is very difficult through the right lobe, because there is limited space for instrument manipulation between the punctured body wall and the CT gantry. In the five cases performed during the pilot trial prior to the main study, we performed the right lobe approach in three cases, as described in previous reports. The result indicated that the investigator suffered from limited space in terms of device manipulation. The space between the right lobe and laterally located CT gantry is too narrow and uncomfortable for the operator to go on the rest steps of only CTF guided PTBD. It may cause contamination and limitation of device manipulation. After changing the puncture target to the left lobe in the two remaining cases of the pilot trial, it was much easier to manipulate instruments and helpful for successful trial.
Of the 28 patients included in the main study, only five underwent the procedure through the right lobe intrahepatic duct (IHD). It was possible in those patients because they had a slim build, securing sufficient space for the procedure between the right abdominal wall and CT gantry. Also, the left lobe puncture has an important advantage in only CTF-guided PTBD. Because segments 5 or 6 IHD is located vertically in the right lobe, it is not easy to monitor the whole course on cross sectional images. On the other hand, segmental ducts in the left lobe have a transverse course, which is easier to trace on CTF. Contrast media injection should be also carefully considered for a trial to be successful. If it is injected immediately after bile duct puncture, it may disturb visualization of guide wire in the bile duct. We injected contrast media after placing a drainage catheter to confirm location of catheter tip.
The present study is not for a comparison of a CTF-only guided PTBD and fluoroscopy-guided PTBD. Fluoroscopy-guided PTBD is definitely the standard method and we are also adopting it in our practice. However, although the clinical status is very grave and symptoms are very severe to tolerate, patients have to wait to undergo a PTBD until the angiography unit is available. Our belief is that if the CTF-guided PTBD is achieved in the CT room, it is comfortable for the patients and helpful to shorten emergency care steps. Of course, if a combination with the C-arm fluoroscopy is possible in the CT unit, it would be very convenient. However, it is usually difficult to arrange a C-arm as a fixed device in CT unit in the aspect of space and economical efficiency. Bringing the C-arm every time would also be troublesome and it might be sometimes impossible.
We evaluated the feasibility and usefulness for the one step CTF guided PTBD. For the best possible success rate, the proper assessment of suitability for this method must be decided upon beforehand. We have to consider both the clinical and anatomical conditions, as described in the inclusion criteria. In particular, the compliance of patients should be carefully considered. Although patients have a large dilated peripheral duct width, patients with respiratory difficulty, uncontrolled motion, severe coughing, and unclear consciousness should be excluded from the indication. Conclusively, although it is not familiar to interventional radiologists, performing one step CTF-guided PTBD is technically feasible with a high success rate in patients determined to be suitable candidates for this procedure. Also, it would be helpful for shortening clinical steps and reducing patient waiting time which is critical due the level of pain endured with the condition.
Figures and Tables
Fig. 1
65-year-old male patient with distal CBD cancer.
A-C. CT scan images show dilated intrahepatic and extrahepatic ducts. It is possible to trace advancement course of guide wire and catheter on CT images (white arrows).
D-F. Monitoring CT fluoroscopy images, segment 3 IHD is punctured and guide wire is subsequently inserted, finally to CBD (dotty arrows).
G. Tip of guide wire is confirmed to be placed in CBD on scanography image.
H, I. Drainage catheter is inserted over guide wire monitoring CT fluoroscopy images (arrows).
J. Cholangiography image is acquired using scanography after finishing procedure.
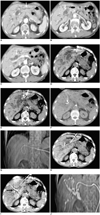
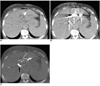
Fig. 2
72-year-old patient with CBD cancer.
A. Using CT fluoroscopy image, left IHD is successfully punctured (bile runs out from needle hub).
B. Next, contrast media is injected to confirm if needle tip is located in bile duct (white arrows).
C. In spite of window setting control, exact identification of guide wire tip was impossible because of highly attenuated contrast media in hilar duct and CBD (arrow).
References
1. Katada K, Anno H, Takeshita G, Ogura Y, Koga S, Ida Y, et al. Development of real-time CT fluoroscopy. Nippon Igaku Hoshasen Gakkai Zasshi. 1994. 54:1172–1174.
2. Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996. 200:851–856.
3. White CS, Templeton PA, Hasday JD. CT-assisted tranbronchial needle aspiration: usefulness of CT fluoroscopy. AJR Am J Roentgenol. 1997. 169:393–394.
4. Froelich JJ, Saar B, Hoppe M, Ishaque N, Walthers EM, Regn J, et al. Real-time CT fluoroscopy for guidance of percutaneous drainage procedures. J Vasc Interv Radiol. 1998. 9:735–740.
5. Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: technique, results and radiation exposure. Radiology. 1999. 212:673–681.
6. Daly B, Krebs TL, Wong-You-Cheong JJ, Wang SS. Percutaneous abdominal and pelvic interventional procedures using CT fluoroscopy guidance. AJR Am J Roentgenol. 1999. 173:637–644.
7. Kirchner J, Kickuth R, Walz MV, Schilling EM, Laufer U, Liermann D. CTF-guided puncture of an unenhanced isodense liver lesion during continuous intravenous injection of contrast medium. Cardiovasc Intervent Radiol. 1999. 22:528–530.
8. Muehlstaedt M, Bruening R, Diebold J, Mueller A, Helmberger T, Reiser M. CT/fluoroscopy-guided transthoracic needle biopsy: sensitivity and complication rate in 98 procedures. J Comput Assist Tomogr. 2002. 26:191–196.
9. Davies RP, Kew J, West GP. Percutaneous jejunostomy using CT fluoroscopy. AJR Am J Roentgenol. 2001. 176:808–810.
10. Ernst RD, Kim HS, Kawashima A, Middlebrook MR, Sandler CM. Near Real-time CT fluoroscopy using computer automated scan technology in nonvascular interventional procedures. AJR Am J Roentgenol. 2000. 174:319–321.
11. Froelich JJ, Wagner HJ, Ishaque N, Alfke H, Scherf C, Klose KJ. Comparison of C-arm CT fluoroscopy and conventional fluoroscopy for percutaneous biliary drainage procedures. J Vasc Interv Radiol. 2000. 11:477–482.
12. Laufer U, Kirchner J, Kickuth R, Adams S, Liermann D. First experiences in CT-guided percutaneous transhepatic biliary decompression by means of real-time CT fluoroscopy. Abdom Imaging. 2001. 26:207–209.
13. Laufer U, Kirchner J, Kickuth R, Adams S, Jendreck M, Liermann D. A comparative study of CT fluoroscopy combined with fluoroscopy versus fluoroscopy alone for percutaneous transhepatic biliary drainage. Cardiovasc Intervent Radiol. 2001. 24:240–244.
14. Kato R, Katada K, Anno H, Suzuki S, Ida Y, Koga S. Radiation dosimetry at CT fluoroscopy: physician's hand dose and development of needle holders. Radiology. 1996. 201:576–578.
15. Stoeckelhuber BM, Leibecke T, Schulz E, Melchert UH, Bergmann-Koester CU, Helmberger T, et al. Radiation dose to the radiologist's hand during continuous CT fluoroscopy-guided interventions. Cardiovasc Intervent Radiol. 2005. 28:589–594.
16. Murphy K, Baez JC, Cooney B, Kabaish K. CT fluoroscopy-guided postmyelographic transthecal radiofrequency ablation of a posterior vertebral body osteoid osteoma. J Vasc Interv Radiol. 2008. 19:291–293.
17. Schaefer PJ, Schaefer FK, Heller M, Jahnke T. CT fluoroscopy guided biopsy of small pulmonary and upper abdominal lesions: efficacy with a modified breathing technique. J Vasc Interv Radiol. 2007. 18:1241–1248.
18. Bladt O, De Wever W. Additional value of CT-fluoroscopic biopsy of pulmonary lesions: a retrospective study of 69 patients. JBR-BTR. 2006. 89:298–302.
19. Hamuro M, Kaminou T, Nakamura K, Matsuoka T, Sakai Y, Morimoto A, et al. Percutaneous ethanol injection under CT fluoroscopy for hypervascular hepatocellular carcinoma following transcatheter arterial embolization. Hepatogastroenterology. 2002. 49:752–757.
20. Tay VK, Fitridge R, Tie ML. Computed tomography fluoroscopy-guided chemical lumbar sympathectomy: simple, safe and effective. Australas Radiol. 2002. 46:163–166.
21. Carlson SK, Bender CE, Classic KL, Zink FE, Quam JP, Ward EM, et al. Benefits and safety of CT fluoroscopy in interventional radiologic procedures. Radiology. 2001. 219:515–529.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download