Abstract
Objective
We wanted to evaluate the MR findings for differentiating between necrotizing fasciitis (NF) and pyomyositis (PM).
Materials and Methods
The MR images of 19 patients with surgically confirmed NF (n = 11) and pathologically confirmed PM (n = 8) were retrospectively reviewed with regard to the presence or absence of any MRI finding criteria that could differentiate between them.
Results
The patients with NF had a significantly greater prevalence of the following MR findings (p < 0.05): a peripheral band-like hyperintense signal in muscles on fat-suppressed T2-weighted images (73% of the patients with NF vs. 0% of the patients with PM), peripheral band-like contrast enhancement (CE) of muscles (82% vs. 0%, respectively) and thin smooth enhancement of the deep fascia (82% vs. 13%, respectively). The patients with PM had a significantly greater prevalence of the following MRI findings (p < 0.05): a diffuse hyperintense signal in muscles on fat-suppressed T2-weighted images (27% of the patients with NF vs. 100% in the patients with PM), diffuse CE of muscles (18% vs. 100%, respectively), thick irregular enhancement of the deep fascia (0% vs. 75%, respectively) and intramuscular abscess (0% vs. 88%, respectively). For all patients with NF and PM, the superficial fascia and muscle showed hyperintense signals on T2-weighted images and CE was seen on fat-suppressed CE T1-weighted images. The subcutaneous tissue and deep fascia showed hyperintense signals on T2-weighted images and CE was seen in all the patients with NF and in seven (88%) of the eight patients with PM, respectively.
Necrotizing fasciitis (NF) is a rapidly progressive, life-threatening illness that's characterized by necrosis and suppuration (1-4). It is often difficult to differentiate NF from cellulitis or pyomyositis (PM) according to the initial clinical presentation (1, 5). However, prompt and aggressive surgical treatment is required to prevent a fatal outcome for patients with NF, whereas other soft tissue infections such as PM or cellulitis do not require emergency surgery (2-6). There have been some reports (2, 7, 8) that have tried to discriminate NF from cellulitis with using MR imaging. To the best of our knowledge, however, there have been only a few reports (2, 9) regarding the differentiation between NF and PM with using MR imaging.
The purpose of this study was to evaluate the MR findings for discriminating between NF and PM.
The ethics committees at our institutions did not require informed patient consent for this retrospective study. MR imaging was performed for 19 patients with clinically suspected NF from July 2003 through June 2007, and the findings were retrospectively reviewed. Eleven surgically proven patients were included in this study. We had 28 patients with clinically suspected PM, although 20 patients among them were excluded due to the absence of cultured organisms. The MR images from the 11 patients with surgically confirmed NF and the eight patients with pathologically confirmed PM from July 2003 through June 2007 were included in this study. All the cases were identified by reviewing the pathologic databases and the operative records. Microorganisms were cultured in seven patients with NF. There were 14 men and 5 women with an age range of 14 to 87 years (mean age: 49 years). The 11 patients with NF were eight men and three women with an age range from 14 to 73 years (mean age: 43 years). The eight patients with PM were six men and two women with an age range from 26 to 87 years (mean age: 58 years).
We also reviewed the clinical manifestations of NF and PM. Nine patients (82%) with NF and five patients (63%) with PM had the following underlying conditions: diabetes mellitus, systemic lupus erythematosus, a history of malignancy or a renal transplantation state. Two patients (18%) with NF were over 60 years old, whereas five patients (63%) with PM were over 60 years old. A history of local trauma was present for three patients (27%) with NF and there was no history of local trauma for the patients with PM.
The MR examinations were performed within 1 to 10 days (median: 5 days, mean: 5.8 days) for the cases of NF, and within 6 to 90 days (median: 14 days, mean: 32.2 days) for the cases of PM after the onset of symptoms, respectively. MR imaging was performed with one of the two 1.5-T MR units (Achieva, Philips Medical Systems, Best, The Netherlands; Twin Speed, GE Medical Systems, Milwaukee, WI). The best fitting coil (body array coil or a shoulder coil) was used for the suspected area of involvement. The variations in the field of view for different sites of involvement ranged from 15 cm to 40 cm for the axial plane, and from 20 cm to 35 cm for the sagittal or coronal plane.
The fat-suppressed (FS) fast spin-echo (FSE) T2-weighted images (3,000-4,600/63-112 [repetition time msec/echo time msec]) and the T1-weighted (450-800/10-21) images were obtained in the axial plane. The T2 weighted spectral fat saturation inversion recovery (SPIR) sequence (3,000-4,600/63-112 [repetition time msec/echo time msec]) was obtained by the Philips scanner, and this was equivalent to the FS FSE T2-weighted sequence obtained by the GE scanner. The FSE T2-weighted images (3,000-4,600/63-112) were obtained for at least one orthogonal plane. For 18 patients, the axial and longitudinal FS T1-weighted spin echo images (450-800/10-21) were obtained after administration of 0.1 mmol per kilogram of body weight of gadopentetate dimeglumine (Magnevist; Bayer Schering, Berlin, Germany). The matrix size was 192-256 × 256 and the number of averaging was one or two. The section thickness and intersection gap were variable for different sites of involvement. The echo train length was 8-16.
The MR images were analyzed on the high-resolution monitors (2,048 × 2,560 matrix, 10-bit viewable gray scale) of a picture archiving and communication system. The MR images were retrospectively reviewed by the consensus of two reviewers who were blinded to the clinical data. Involvement of the superficial and deep fasciae, muscles and subcutaneous tissue, the pattern of muscle involvement and the presence of intramuscular abscess was assessed, as well as the CE pattern of the fascia and muscle.
The pattern of an abnormal signal or the CE of muscle was classified as peripheral band-like or diffuse. An abnormal muscle signal or CE was classified as peripheral band-like if it was particularly prominent along the fascia, despite being accompanied with some diffuse abnormal signal within the muscle. We classified an abnormal signal or the CE of muscle as diffuse if it was diffusely observed within the muscle without a prominent abnormal signal along the fascia. On the FS CE T1-weighted images, the CE pattern of fascia was assessed as follows: thin smooth or thick irregular. Muscle involvement was assessed in terms of the anatomical compartments. If an abnormal muscle signal was observed in three or more anatomical compartments on the axial FS T2-weighted images and the FS CE T1-weighted images, then it was regarded as multicompartment involvement.
The patients with either NF or PM complained of nonspecific symptoms that included painful swelling, tenderness or a local heat sensation. Three patients (27%) with NF presented with bullae formation on their skin. NF and PM both affected the lower extremities more frequently than upper extremities: lower leg (n = 5), buttocks through thigh (n = 3), arm (n = 2) and thigh (n = 1) for the NF patients; buttocks (n = 2), buttocks through thigh (n = 2), lower leg (n = 2), thigh (n = 1) and upper arm (n = 1) for the PM patients. Ten patients (91%) with NF showed multi-compartmental involvement except for one patient with single-compartmental involvement. Five patients (63%) with PM showed single-compartmental involvement, and three other PM patients showed multi-compartmental involvement. Unilateral involvement was observed in all the patients with either NF or PM.
The causative organisms in the patients with NF were as follows: streptococcus pyogenes (n = 2), polymicrobial organisms (n = 2), staphylococcus aureus (n = 1), streptococcus group F (n = 1), mycobacterium species (n = 1) or no growth (n = 2). On the other hand, staphylococcus aureus (n = 6) was the most common causative organism in the eight patients with PM (Table 1).
Patients with NF had a significantly greater prevalence of the following MR findings (p < 0.05): peripheral band-like hyperintense signals in muscles on the FS T2-weighted images (73% for the NF patients vs. 0% for the PM patients), peripheral band-like CE of muscles (82% vs. 0%, respectively), thin smooth enhancement of deep fascia (82% vs. 13%, respectively) and multicompartment involvement (91% vs. 38%, respectively) (Table 2) (Figs. 1, 2).
Patients with PM had a significantly greater prevalence of the following MR findings (p < 0.05): diffuse hyperintense signals in the involved muscles on the FS T2-weighted images (27% in the NF patients vs. 100% in the PM patients), diffuse CE of the involved muscles (18% vs. 100%, respectively), thick and irregular enhancement of deep fascia (0% vs. 75%, respectively) and intramuscular abscess formation (0% vs. 88%, respectively) (Table 2) (Fig. 3).
In all the patients with NF and PM, the superficial fascia and muscles showed hyperintense signals on the T2-weighted images and CE was seen on the FS CE T1-weighted images. The subcutaneous tissue and deep fascia showed hyperintense signals on the T2-weighted images, and CE was seen on the FS CE T1-weighted images in all the patients with NF and in seven (88%) of the eight patients with PM.
The number of patients with NF has increased during the recent decades, and this is possibly due to a recent mutation of a subtype of group A beta-hemolytic Streptococci (10, 11). PM is a primary bacterial infection involving the skeletal muscles (4, 9, 12). Although NF can expand rapidly through the facial planes and along the underlying muscles, the overlying skin often resembles that of patients afflicted with cellulitis or PM (2). NF can only be confirmed during surgery: extensive undermining of the surrounding tissue is typically observed and the facial plane lacks resistance to a blunt instrument (7, 13).
Our prevalence (100%) of an abnormal signal in the muscle of NF patients was consistent with the previous report by Schmid et al. (7) that an intramuscular increased signal was seen in 10 of 11 cases of NF and intramuscular CE was seen in seven of nine cases with NF, respectively. However, our prevalence was much higher than the prevalence reported in a study by Rahmouni et al. (14) that seven (54%) of 13 MR images of the patients with necrotizing soft-tissue infections showed poorly defined areas of hyperintensity within muscles on the T2-weighted images. This difference could be related to the fact that we added the fat-suppression technique to the CE T1-weighted imaging as well as to the FSE T2-weighted imaging. On the other hand, our prevalence (100%) of an abnormal signal in muscle in PM patients was consistent with the previous report by Gordon et al. (12) that an intramuscular increased signal was seen on the T2-weighted images of all 11 patients with PM.
In particular, we found that patients with NF showed a peripheral band-like hyperintense signal in muscles on the FS T2-weighted images and peripheral band-like CE in muscles on the FS CE T1-weighted images, and this was in contrast to the PM patients who had a diffuse hyperintense signal on the FS T2-weighted images, and diffuse CE in muscles. The difference between NF and PM may stem from the fact that the focus of NF disease is fascia, while the focus of PM disease is muscle. NF patients often present with multi-compartmental involvement, while most cases of PM present with single-compartmental involvement.
A peripheral band-like abnormal signal in our patients with NF is in contrast to the previous report (7) that the intramuscular signal was diffusely increased on the T2-weighted images and it showed diffuse CE. We assume that this discrepancy is related to the different criteria of analysis. In this present study, an abnormal signal or CE of muscle was classified as peripheral band-like instead of diffuse if it was particularly prominent along the fascia, and this was despite being accompanied by some diffuse abnormal signal within the muscle. Intramuscular abscess was not observed in our NF patients and this is in sharp contrast to the previous report (7) that abscesses were identified in six among the nine cases of NF. Thus, a further study with a larger study population may be needed.
Liquefaction tissue necrosis and inflammatory edema both contribute to the accumulation of fluid in the fascia (15, 16). Indeed, in each of our NF subjects, the superficial and deep fasciae showed a hyperintense signal on the T2-weighted images and CE was seen, like that of a prior report (7). However, such an abnormal signal and enhancement of the deep fascia were not unique findings for NF, which is consistent with other previous studies (3, 17). Instead, in this present study, the enhancement pattern of the deep fascia was significantly different for the two patient groups. Most patients (82%) with NF showed thin smooth CE of the deep fascia, while most patients (75%) with PM showed thick irregular enhancement of this area.
The major limitations of this study include that the MR reviewers knew that all the patients had confirmed NF or PM. This may have increased the sensitivity of detecting each of the MR findings. Another limitation is the small number of patients, and particularly the number of patients with PM. This could be related to the fact that only pathologically confirmed patients with PM and who had MR imaging were included in this study, although the prevalence of PM is much higher than the prevalence of NF. One other limitation of our study was that the study was confined to infectious conditions such as NF and PM, because we excluded such noninfectious conditions as idiopathic inflammatory myopathy, noninfectious inflammatory eosinophilic fasciitis, rheumatic diseases, phlebedema, lymphedema, exertional muscle injury and other tumorous conditions such as lymphoma. Thus, other conditions except for PM might have MR findings that are similar to those of NF.
In conclusion, the muscle involvement pattern on the FS T2-weighted images, as well as the enhancement pattern of muscle and fascia on the FS CE T1-weighted images, can help differentiate NF from PM.
Figures and Tables
Fig. 1
51-year-old man with surgically confirmed necrotizing fasciitis.
A. Axial fat-suppressed T2-weighted image (TR/TE, 4,000/87) shows peripheral band-like high signal intensity (arrows) in involved muscles of anterior, lateral and posterior compartments of left lower leg. Hyperintense signal is seen along deep fascia (arrowheads) as well as along superficial fascia.
B, C. Axial T1-weighted image (TR/TE, 450/12) (B) and fat-suppressed contrast-enhanced T1-weighted image (550/12) (C) show peripheral band-like enhancement (arrows) of involved muscles. Thin smooth enhancement is seen along deep fascia (arrowheads) as well as along superficial fascia. Patient underwent emergency fasciectomy. Streptococcus pyogenes was cultured.
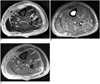
Fig. 2
37-year-old man with surgically confirmed necrotizing fasciitis.
A. Axial fat-suppressed T2-weighted image (TR/TE, 4,400/81) shows peripheral band-like high signal intensity (arrows) in involved muscles of anterior, medial and posterior compartments of left thigh. Hyperintense signal is seen along deep fascia (arrowheads) as well as along superficial fascia.
B, C. Axial T1-weighted image (TR/TE, 450/15) (B) and fat-suppressed contrast-enhanced T1-weighted image (600/15) (C) show peripheral band-like enhancement (arrows) of involved muscle and thin smooth enhancement of deep fascia (arrowheads). Patient underwent emergency fasciectomy. Streptococcus pyogenes was cultured.
D. Histopathologic specimen reveals marked neutrophilic and histiocytic infiltrates and necrosis of fascia (Hematoxylin & Eosin staining; original magnification, ×200).
E. Photomicrograph of histologic specimen shows severe inflammatory changes and necrosis in muscle (Hematoxylin & Eosin staining; original magnification, ×100). Patient underwent emergency fasciectomy. Streptococcus pyogenes was cultured.
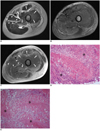
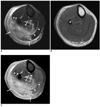
Fig. 3
29-year-old woman with pathologically confirmed pyomyositis.
A. Axial fat-suppressed T2-weighted image (TR/TE, 3,200/96) shows diffuse hyperintense signal (arrows) in soleus and lateral head of gastrocnemius muscles, and this is confined to posterior compartment of lower leg. Streaky hyperintense signal is seen in subcutaneous tissue. Hyperintense signal is seen along deep fascia (arrowheads) as well as along superficial fascia.
B, C. Axial T1-weighted image (TR/TE, 480/17) (B) and axial fat-suppressed contrast-enhanced T1-weighted image (784/17) (C) shows diffuse enhancement (arrows) of involved muscles and subcutaneous tissue. Thick irregular enhancement is seen along deep fascia (arrowheads) as well as along superficial fascia. Staphylococcus aureus was cultured. Patient recovered after antibiotic therapy.
References
1. Wilson B. Necrotizing fasciitis. Am J Surg. 1952. 18:416–431.
2. Brothers TE, Tagge DU, Stutley JE, Conway WF, Del Schutte H Jr, Byrne TK. Magnetic resonance imaging differentiates between necrotizing and non-necrotizing fasciitis of the lower extremity. J Am Coll Surg. 1998. 187:416–421.
3. Arslan A, Pierre-Jerome C, Borthne A. Necrotizing fasciitis: unreliable MRI findings in the preoperative diagnosis. Eur J Radiol. 2000. 36:139–143.
4. McAllister L. Kransdorf MJ, Murphey MD, editors. Masses that may mimic soft tissue tumors. Imaging of soft tissue tumors. 2006. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;511–572.
5. Wall DB, de Virgilio C, Black S, Klein SR. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg. 2000. 179:17–21.
6. Beauchamp NJ Jr, Scott WW Jr, Gottlieb LM, Fishman EK. CT evaluation of soft tissue and muscle infection and inflammation: a systematic compartmental approach. Skeletal Radiol. 1995. 24:317–324.
7. Schmid MR, Kossmann T, Duewell S. Differentiation of necrotizing fasciitis and cellulitis using MR imaging. AJR Am J Roentgenol. 1998. 170:615–620.
8. Duewell S, Hagspiel KD, Zuber J, von Schulthess GK, Bollinger A, Fuchs WA. Swollen lower extremity: role of MR imaging. Radiology. 1992. 184:227–231.
9. Revelon G, Rahmouni A, Jazaerli N, Godeau B, Chosidow O, Authier J, et al. Acute swelling of the limbs: magnetic resonance pictorial review of fascial and muscle signal changes. Eur J Radiol. 1999. 30:11–21.
10. Martin DR, Single LA. Molecular epidemiology of group A streptococcus M type 1 infections. J Infect Dis. 1993. 167:1112–1117.
11. Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996. 334:240–245.
12. Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology. 1995. 197:279–286.
13. Stamenkovic I, Lew PD. Early recognition of potentially fatal necrotizing fasciitis. The use of frozen-section biopsy. N Engl J Med. 1984. 310:1689–1693.
14. Rahmouni A, Chosidow O, Marthieu D, Gueorguieva E, Jazaerli N, Radier C, et al. MR imaging in acute infectious cellulitis. Radiology. 1994. 192:493–496.
15. Struk DW, Munk PL, Lee MJ, Ho SG, Worsley DF. Imaging of soft tissue infections. Radiol Clin North Am. 2001. 39:277–303.
16. Fugitt JB, Puckett ML, Quigley MM, Kerr SM. Necrotizing fasciitis. Radiographics. 2004. 24:1472–1476.
17. Loh NN, Ch'en IY, Cheung LP, Li KC. Deep fascial hyperintensity in soft-tissue abnormalities as revealed by T2-weighted MR imaging. AJR Am J Roentgenol. 1997. 168:1301–1304.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download