Abstract
Objective
To determine the accuracy of CT and positron emission tomography (PET) in the diagnosis of recurrent uterine cervical cancer.
Materials and Methods
Imaging findings of CT and PET in 36 patients (mean age, 53 years) in whom recurrent uterine cervical cancer was suspected were analyzed retrospectively. Between October 1997 and May 1998, they had undergone surgery and/or radiation therapy. Tumor recurrence was confirmed by pathologic examination or follow-up studies.
Results
In detecting recurrent uterine cervical cancer, the sensitivity, specificity, and accuracy of CT were 77.8%, 83.3%, and 80.5%, respectively, while for PET, the corresponding figures were 100%, 94.4%, and 97.2%. The Chi-square test revealed no significant difference in specificity (p = .2888), but significant differences in sensitivity (p = .0339) and accuracy (p = .0244).
The recurrence rate of uterine cervical cancer is reported to be 6.5% after surgery and 26.2% after radiation therapy alone (1). Radiological studies such as intravenous urography (IVU), US, CT, and MR imaging are used to detect recurrent cervical cancer (2). It is difficult, however, for these imaging modalities to differentiate recurrent tumor from postoperative or radiation fibrosis, and to detect metastatic normal-sized lymph nodes and extrapelvic metastases (2-7).
Since Di Chiro et al. (8) first used it to detect recurrent brain tumors, positron emission tomography (PET), the diagnostic modality which makes use of increased glycolysis in tumor cells, has been used to detect recurrent tumors in many organs.
To our knowledge, no report has described the diagnosis of recurrent cervical cancer using PET. The purpose of this study is to compare the accuracy of CT with that of PET in the detection of recurrent cervical cancer.
Among patients with uterine cervical cancer who had undergone initial treatment between October 1997 and May 1998, CT and PET were performed in 36 in whom recurrence was clinically suspected. As initial treatment, 13 patients had undergone surgery alone, 14 radiation therapy alone, and nine surgery and postoperative radiation therapy. Recurrence was suspected on the basis of increased levels of serum squamous cell carcinoma antigen (SCCA) and carcinoembryonic antigen (CEA), pain in the lower abdomen and back, edema of the lower leg, and oliguria.
All patients underwent CT and PET, the former involving the use of a GE CT/i 9800 scanner (General Electric Medical Systems, Milwaukee, WI), with 10mm thickness. Images of the chest, abdomen, and pelvis were obtained after intravenous injection of 150 ml non-ionic contrast media. For PET, 18F-FDG (2-[fluorine-18]fluoro-2-deoxy-D-glucose) with a GE Advance scanner (General Electric Medical Systems, Milwaukee, WI) was used. During the six hours prior to scanning, patients were restricted to orally and intravenously administered glucose. In the PET room, 10mCi of FDG was administered intravenously prior to intravenous hydration with one liter of normal saline. Thirty minutes after the administration of FDG, Lasix 20 mg was intravenously injected. To avoid artifactual accumulation of FDG in the urinary bladder, a Foley catheter with drainage bag was then positioned.
Two radiologists (DHP, KHK) and one nuclear medicine physician (CWC) retrospectively analyzed the imaging findings. As seen on CT, definite metastatic mass or a nodule and lymph node larger than 1 cm along the short axis were interpreted as positive findings. On PET, we interpreted a high metabolic area of over 2.5 ml/kg of SUV (standardized uptake value; mean activity of region of interest [mCi/ml]/injected dose [mCi/ ml]/body weight[kg]) as a positive finding. Recurrence was confirmed by percutaneous lymph node biopsy in ten patients, biopsy of the pelvic mass in three and by follow-up study in 23. Tumor marker study and CT at 3- and 6-month intervals were used for follow up, which in most patients lasted for 18 to 24 months. Where either 1) increased tumor marker, 2) increased size of masses or lymph nodes, as seen on CT, or 3) decreased size of masses and lymph nodes after radiation therapy and chemotherapy was noted, it was considered that the condition had recurred.
In addition, the location and extension of recurrent masses or metastatic lymph nodes were analyzed for surgical extirpation and determination of radiation portal.
In 18 patients, recurrence was confirmed by pathologic examination and follow-up study. CT revealed three false-positive cases and four false negatives, while on PET there was only one false positive. On CT, sensitivity, specificity, and accuracy were 77.8%, 83.3%, and 80.5%, respectively, while for PET, the corresponding figures were 100%, 94.4%, and 97.2% (Table 1).
The three false-positive cases interpreted as recurrence of soft tissue masses in the pelvic cavity and seen on CT showed no high metabolic area on PET, and were shown by follow-up study to be postoperative or radiation fibrosis (Fig. 1). The four false-negative cases seen on CT and interpreted as no recurrence were lymph nodes smaller than 1 cm in the abdomen, pelvic cavity, and inguinal area (Fig. 2). Unfortunately, however, they showed a high metabolic area and were confirmed by follow-up percutaneous biopsy or increased size as recurrence. The one false-positive case thought because of the high metabolic area seen on PET in the left upper lung field to be recurrence, was shown by follow-up study and sputum polymerase chain reaction to be tuberculosis (Fig. 3).
Due to poor spatial resolution, PET failed to detect the extent of recurrent pelvic masses and the exact level of lymph nodes. To determine the possibilities of surgery and the required extent of radiation therapy, CT was therefore needed.
About half of all cases of recurrent uterine cervical cancer are confined to the pelvic cavity, but some cases show metastatic lesions in the lymph nodes, lung, bone, and liver (9). Halpin et al. (1) reported that where there is recurrence, 60 per cent of cases are diagnosed within two years, and 82 per cent within four years. Eighty percent of patients in whom there was recurrence died within one year, and ninety percent within two years.
The treatment of recurrent uterine cervical cancer differs according to the extent of the recurrent lesion. If this is confined to the pelvic cavity, pelvic exenteration is the treatment of choice. On the other hand, if it recurs beyond the pelvic cavity, radiation therapy or chemotherapy is preferred. The detection and exact localization of a recurrent lesion are therefore very important (10).
Clinically, patients with recurrence complain of back pain, sciatic pain, and edema of the lower leg, but some show no symptoms or signs of recurrent cervical cancer (1, 2). Radiologic imaging studies such as IVU, barium enema, lymphangiography, and US are used to detect recurrent uterine cervical cancer, but the findings are indirect (2).
In cases of recurrent cervical cancer, CT scanning demonstrates a pelvic mass or enlarged pelvic and para-aortic lymph nodes (2). The extent of a tumor, and lymphadenopathy and hydronephrosis, can be easily detected on CT (2). CT scanning is also helpful in determining the radiation portal, the site for biopsy, and the effect of treatment. Consequently, the modality has been used as a gold standard in the diagnosis of recurrent uterine cervical cancer. With CT scanning, however it may be difficult to differentiate recurrence from postoperative and postradiation fibrosis, and to detect normal-sized metastatic lymph nodes (2, 3). On MR images, a recurrent lesion shows increased signal intensity on T2-weighted images, but reports of its specificity and sensitivity have varied. In addition, MR cannot distinguish necrosis, edema, hemorrhage and inflammation from recurrence (4-7).
PET with FDG, which makes use of increased glycolysis levels in tumor cells, is a noninvasive diagnostic method used in functional imaging of the tumor, and has been used to detect primary tumors and recurrence, to determine the efficacy of therapy, for staging, and to detect the extent of a tumor (11-25). Ogunbiyi et al. (20) compared PET with CT in the detection of recurrent or metastatic colon and rectal cancer. They reported that PET accurately detected met-astatic lesions as well as local recurrences, and was especially effective in differentiating between recurrence and postoperative fibrosis. Anzai et al. (12) compared PET with CT and MR in recurrent head and neck cancer after surgery or radiation therapy, and reported that local hematoma, abscess, fistula, and reconstruction flap could not be clearly differentiated from local recurrence. They also reported that sensitivity and specificity were 88% and 100% with PET, but 25% and 75% with CT and MR, respectively. Hudgins et al. (26) reported that in head and neck cancer, MR could not easily distinguish between postradiation fibrosis and recurrence. McGuirt et al. (18) also reported that PET was superior to CT in terms of sensitivity and specificity, and that if PET revealed no areas of high metabolism, pathologic examination could be delayed.
PET is superior to CT in detecting small metastatic lymph nodes, especially in patients in whom the fat content of the retroperitoneum is low. Thus, PET is effective in distinguishing metastatic lymph nodes from testicular cancer, lymphoma, and rectal and cervical cancer, which prefer to metastasize to retroperitoneal lymph nodes. PET can also differentiate recurrence from scar tissue, irrespective of anatomical alteration and surgical clip (27). With CT, we also experienced difficulty in differentiating recurrence from postoperative and postradiation fibrosis and in detecting small lymph nodes. PET can, in addition, be used to obtain whole body images and detect recurrence that was not clinically suspected. A disadvantage of PET is its high cost; another is that it does not easily determine anatomical location and tumor extent. Using PET, we were unable to decide the exact location of a recurrent mass and the extent of invasion of an adjacent organ. Eventually, therefore, in order to decide the treatment plans of patients in whom recurrence was detected, CT was required.
In conclusion, PET is a reliable diagnostic screening modality. It can detect small lymph nodes, distinguish between recurrence and fibrosis, and can be used for whole-body scanning. Tumor localization is not one of its strengths, however, and in order to determine a treatment plan, CT scanning is therefore necessary.
Figures and Tables
Fig. 1
A 39-year-old woman who had undergone radical hysterectomy due to uterine cervical carcinoma.
A. Enhanced CT scan shows soft tissue mass on the left pelvic side wall (arrow).
B. PET scan shows no hypermetabolic site in the pelvis. Since no interval change was seen during follow-up study, we concluded that this was a case of postoperative fibrosis. The highly metabolic lesion in left abdomen (arrow) is due to artifactual accumulation of FDG in ascending colon.
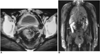
Fig. 2
A 52-year-old woman who had undergone radiation therapy due to uterine cervical cancer.
A. Enhanced CT scan shows lymph node smaller than 1cm in the para-aortic area (arrow).
B. PET scan shows hypermetabolic area (SUV = 3.8 ml/kg) in the para-aortic chain (arrow).
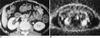
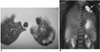
Fig. 3
A 41-year-old woman with uterine cervical cancer. False positive PET scan due to pulmonary tuberculosis.
A. CT scan shows multiple nodules in left upper lung (arrows).
B. PET scan shows hypermetabolic lesion (SUV = 9.6 ml/kg) in left upper lung (arrow). Tuberculosis was confirmed by polymerase chain reaction of the sputum.
References
1. Halpin TF, Frick HC, Munnell EW. Critical points of failure in the therapy of cancer of the cervix: a reappraisal. Am J Obstet Gynecol. 1972. 114:755–764.
2. Walsh JW, Amendola MA, Hall DJ, Tisnado J, Goplerud DR. Recurrent carcinoma of the cervix: CT diagnosis. AJR. 1981. 136:117–122.
3. Cunningham JJ, Fuks ZY, Castellino RA. Radiographic manifestations of carcinoma of the cervix and complications of its treatment. Radiol Clin North Am. 1974. 12:93–108.
4. Weber TM, Sostman HD, Spritzer CE, et al. Cervical carcinoma: determination of recurrent tumor extent versus radiation changes with MR imaging. Radiology. 1995. 194:135–139.
5. Hricak H, Swift PS, Campos Z, Quivey JM, Gildengorin V, Goranson H. Irradiation of the cervix uteri: value of unenhanced and contrast-enhanced MR imaging. Radiology. 1993. 189:381–388.
6. Williams MP, Husband JE, Heron CW, Cherryman GR, Koslin DB. Magnetic resonance imaging in recurrent carcinoma of the cervix. Br J Radiol. 1989. 62:544–550.
7. Flueckiger F, Ebner F, Poschauko H, Tamussino K, Einspieler R, Ranner G. Cervical cancer: serial MR imaging before and after primary radiation therapy-a 2-year follow-up study. Radiology. 1992. 184:89–93.
8. Di Chiro G. Positron emission tomography using [18F]fluorodeoxyglucose in brain tumors-a powerful diagnostic and prognostic tool. Invest Radiol. 1988. 22:360–371.
9. Carlson V, Delclos L, Fletcher GH. Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology. 1967. 88:961–966.
10. Keettel WC, Van Voorhis LW, Latourette HB. Management of recurrent carcinoma of the cervix. Am J Obstet Gynecol. 1968. 102:671–677.
11. Hoh CK, Schiepers C, Seltzer MA, et al. PET in oncology: will it replace the other modalities? Semin Nucl Med. 1997. 27:94–106.
12. Anzai Y, Carroll WR, Quint DJ, et al. Recurrence of head and neck cancer after surgery or irradiation: prospective comparison of 2-deoxy-2-[F-18]fluoro-D-glucose PET and MR imaging diagnoses. Radiology. 1996. 200:135–141.
13. Bender H, Kirst J, Palmedo H, et al. Value of 18-fluoro-deoxyglucose positron emission tomography in the staging of recurrent breast carcinoma. Anticancer Res. 1997. 17:1687–1692.
14. Budinger TF, Brennan KM, Moses WW, Derenzo SE. Advances in positron tomography for oncology. Nucl Med Biol. 1996. 23:659–667.
15. Blahd WH, Brown CV, Khonsary SA, et al. PET scans of abdominal malignancy. World J Surg. 1996. 20:245–247.
16. Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK Jr, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg. 1998. 133:510–516.
17. Haseman MK, Reed NL, Rosenthal SA. Monoclonal antibody imaging of occult prostate cancer in patients with elevated prostate-specific antigen. Positron emission tomography and biopsy correlation. Clin Nucl Med. 1996. 21:704–713.
18. McGuirt WF, Greven KM, Keyes JW Jr, Williams DW 3rd, Watson N. Laryngeal radionecrosis versus recurrent cancer: a clinical approach. Ann Otol Rhinol Laryngol. 1998. 107:293–296.
19. Miraldi F, Vesselle H, Faulhaber PF, Adler LP, Leisure GP. Elimination of artifactual accumulation of FDG in PET imaging of colorectal cancer. Clin Nucl Med. 1998. 23:3–7.
20. Ogunbiyi OA, Flanagan FL, Dehdashti F, et al. Detection of recurrent and metastatic colorectal cancer: comparison of positron emission tomography and computed tomography. Ann Surg Oncol. 1997. 4:613–620.
21. Greven KM, Williams DW 3rd, Keyes JW Jr, McGuirt WF, Watson NE Jr, Case LD. Can positron emission tomography distinguish tumor recurrence from irradiation sequelae in patients treated for larynx cancer? Cancer J Sci Am. 1997. 3:353–357.
22. Garner CM. Positron emission tomography: new hope for early detection of recurrent brain tumors. Cancer Nurs. 1997. 20:277–284.
23. Schiepers C. Role of positron emission tomography in the staging of lung cancer. Lung Cancer. 1997. 17(1):S. 29–35.
24. Simon GH, Nitzsche EU, Laubenberger JJ, Einert A, Moser E. PET imaging of recurrent medullary thyroid cancer. Nuklearmedizin. 1996. 35:102–104.
25. Keogan MT, Lowe VJ, Baker ME, McDermott VG, Lyerly HK, Coleman RE. Local recurrence of rectal cancer: evaluation with F-18 fluorodeoxyglucose PET imaging. Abdom Imaging. 1997. 22:332–337.
26. Hudgins PA, Burson JG, Gussack GS, Grist WJ. CT and MR appearance of recurrent malignant head and neck neoplasms after resection and flap reconstruction. AJNR. 1994. 15:1689–1694.
27. Vesselle HJ, Miraldi FD. FDG PET of the retroperitoneum: normal anatomy, variants, pathologic conditions, and strategies to avoid diagnostic pitfalls. RadioGraphics. 1998. 18:805–823.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download