Abstract
Objective
To describe the neuroradiologic findings of primary antiphospholipid antibody syndrome (PAPS).
Materials and Methods
During a recent two-year period, abnormally elevated antiphospholipid antibodies were detected in a total of 751 patients. In any cases in which risk factors for stroke were detected - hypertension, diabetes mellitus, hyperlipidemia, smoking, and the presence of SLE or other connective tissue diseases - PAPS was not diagnosed. Neuroradiologic studies were performed in 11 of 32 patients with PAPS. We retrospectively reviewed brain CT (n = 7), MR (n = 8), and cerebral angiography (n = 8) in 11 patients with special attention to the presence of brain parenchymal lesions and cerebral arterial or venous abnormalities.
Results
CT or MR findings of PAPS included nonspecific multiple hyper-intensity foci in deep white matter on T2-weighted images (5/11), a large infarct in the territory of the middle cerebral artery (4/11), diffuse cortical atrophy (2/11), focal hemorrhage (2/11), and dural sinus thrombosis (1/11). Angiographic findings were normal (5/8) or reflected either occlusion of a large cerebral artery (2/8) or dural sinus thrombosis (1/8).
In 1986, Harris et al. (1) proposed criteria for a new clinical syndrome characterized by unexplained recurrent venous or arterial thrombosis, fetal loss occuring more than twice, or thrombocytopenia in the presence of autoantibodies to phospholipids such as anticardiolipin antibody and lupus anticoagulant. Approximately one half of patients with antiphospholipid antibody syndrome do not have associated systemic lupus erythematosus (SLE) or other collagen vascular disease, and such cases were classified as primary antiphospholipid antibody syndrome (PAPS) (1-4). During the past ten years, it has become clear that the thrombosis associated with antiphospholipid antibodies is a major cause of organ damage in PAPS, SLE and other autoimmune diseases (5). Early descriptions of PAPS were published largely in the rheumatology literature.
Cases involving variable sized infarctions, diffuse cortical atrophy, white matter abnormality, and dural sinus thrombosis in patients with PAPS have been reported in the radiologic literature (6, 7). In this study, we reviewed the neuroradiologic findings in 11 Korean patients in whom PAPS was diagnosed, and compared our observations with previous reports.
We reviewed the results of laboratory tests for the presence of lupus anticoagulant and anticardiolipin antibody performed between May 1995 and May 1997. The lupus anticoagulant test included prothrombin time, active partial thromboplastin time, the tissue thromboplastin inhibition test, thrombin clot time, and a diluted Russell viper venom time. Anticardiolipin antibody (IgG and IgM) determination involved the use of enzyme-linked immunosorbent assay (ELISA). Antiphospholipid antibodies were detected in 751 of 3250 tested patients. Of these 751, 361 showed elevated anticardiolipin levels, 239 showed abnormal lupus anticoagulants, and the remaining 151 showed elevation of both antibodies. We used the diagnostic criteria proposed by Harris et al. (1, 2), which include recurrent thrombosis, more than two spontaneous abortions, and elevated lupus anticoagulant or anticardiolipin antibody on two occasions more than eight weeks apart. When patients fulfilled the Harris criteria and had no other risk factors for vascular thrombosis or occlusion (hypertension, diabetes mellitus, hyperlipidemia, smoking, SLE or other connective tissue diseases), PAPS was diagnosed. Patients with elevated lupus anticoagulant or anticardiolipin antibody and other risk factors for vascular thrombosis or occlusion were excluded, and PAPS was confirmed in the 32 patients who remained. Eleven of these underwent neuroradiologic studies to evaluate symptoms such as hemiplegia (n = 6), headache (n = 3), dementia (n = 1), and memory disturbance (n = 1).
Brain CT was performed in seven patients according to our routine brain CT protocol: slice thickness, 5-10 mm; FOV, 210 mm; standard alogorithm; scan time, 2 sec. Using a 1.5-T system (General Electric Medical Systems, Milwau-kee, WI, version 5.4), brain MR was performed in eight patients according to our routine brain MR protocol: axial and sagittal spin-echo T1-weighted images, axial fast spin-echo T2-weighted images (TR/TE, 420/14 msec for T1-weighting, 3500/99 msec for T2-weighting; slice thickness/gap, 6-7/2.4-2.8 mm; FOV, 200-250 mm; matrix size, 256 or 512). In four patients, axial and coronal T1-weighted images were obtained after intravenous injection of gadolinium-DTPA (0.1 mmol/kg). In one patient, fluid attenuated inversion recovery images (FLAIR; TR/TI/TE, 9999/2500/119 msec) were obtained in addition to fast spin-echo T2-weighted images. To evaluate cerebral vascular status of eight patients, cerebral angiography was performed using a digital subtraction biplane angiography system (Integris BN 3000; Philips Medical Systems, Eindhoven, Netherlands) via transfemoral route.
The 11 patients comprised five men and six women whose ages ranged between 20 and 60 (mean, 39) years. Imaging studies were retrospectively reviewed by two radiologists (JHK, CGC), who worked together. CT and MR studies were reviewed, with special attention to the signal or attenuation characteristics and location of lesions, and presence of hemorrhage. Angiographic studies were reviewed with special attention to obstruction or filling defects in the intracranial vessels.
The symptoms and imaging features of the 11 patients are summarized in Table 1. Six showed acute neurologic symptoms and signs of hemiplegia while the other 5 complained of chronic neurologic symptoms such as headache, dementia, sensory change, and general weakness. Abnormal brain CT findings were a large territorial infarct (3/7), low densities of periventricular white matter (2/7), focal hemorrhage in the basal ganglia (1/7), and dural sinus thrombosis (1/7). MR findings were multiple hyper-intensity foci in the deep white matter on T2-weighted images (5/8), a large territorial infarct (2/8), diffuse cortical atrophy (2/8), focal hemorrhage in the basal ganglia (1/8), and dural sinus thrombosis (1/8). Angiographic findings were normal (5/8), or reflected either occlusions of the middle cerebral artery (2/8) or dural sinus thrombosis (1/8).
Multiple hyper-intensity foci in the deep white matter seen on T2-weighted MR images or low densities in the periventricular white matter seen on CT were the most common findings, and were detected in five of eleven patients (Fig. 1). Cerebral angiography was normal in four of these five patients. A large infarct in the territory of the middle cerebral artery seen on CT and MR was the second most common findings (4/11). In two of such patients, abrupt cut-off of the corresponding middle cerebral arteries was detected by cerebral angiography (Fig. 2), though echocardiography revealed no risk of cardiogenic embolism. MR demonstrated diffuse cortical atrophy in two patients (Fig. 3), and in these two, T2-weighted MR images showed multiple hyper-intensity foci in the deep white matter. Cerebral angiography, performed in one of these two patients, demonstrated normal findings. Focal hemorrhage in the basal ganglia was detected in two patients. In one patient with a focal hemorrhagic and edematous lesion in left rolandic area, CT, MR and angiography indicated the presence of dural sinus thrombosis (Fig. 4). In this patient, cerebral angiography revealed filling defects in the superior sagittal, right transverse, and sigmoid sinus.
In our study, multiple small lesions in the deep white matter were the most common findings. Multiple hyper-intensity foci in the deep white matter seen on T2-weighted MR images or low densities in the periventricular white matter revealed by CT are nonspecific findings. These abnormalities are similar to those seen in many demyelinating, inflammatory, or vascular diseases of the brain such as multiple sclerosis, SLE, Behcet's disease, Sjogren's syndrome, migraine, and small vessel atherosclerotic disease.
Provenzale et al. (6, 7) reported multiple small lacunar infarcts or white matter abnormalities as one of the most common findings in patients with brain involvement of PAPS. Toubi et al. (8) found antiphospholipid antibody immunoreactivity in 55% of SLE patients with CNS manifestation. In his study, 62% of neuro-SLE patients with positive antiphospholipid antibody immunoreactivity showed abnormal hyper-intensity in the white matter on T2-weighted MR imaging, and this was interpreted as 'suggestive of vasculopathy'. These data suggest the possibility of a pathogenic link between abnormal white matter lesions and antiphospholipid antibodies, though the exact mechanism of tissue injury is not known. Thrombotic occlusion of small arterioles or venules in the brain, with characteristic hypercoagulability of the blood, is a plausible explanation. The other possible mechanism is a non-vascular or non-ischemic one, such as direct interaction between antiphospholipid antibodies and cell membrane phospholipid. Currently, there is no direct evidence to support these hypotheses but thrombotic occlusion of cerebral vessels is regarded as the most likely mechanism of tissue injury in PAPS (8-11).
A large infarct in the territory of the middle cerebral artery was the second most common finding in our small series and it may occur due to the thrombosis of cerebral arteries or embolism from cardiac valvular lesions associated with PAPS. Arterial thrombosis may be a major cause of territorial infarct in patients with PAPS (12). Lechner and Pabinger-Fasching (13) reported a higher prevalence of arterial thrombosis (25%) in the brain than in other parts of body (16%), and the special predilection of PAPS for cerebral arterial thrombosis is now accepted (13, 14). In other parts of the body, arterial thrombosis appears to be less prevalent than venous thrombosis which accounts for more than 70% of PAPS-related thromboses. In the brain, however, arterial thrombosis is more common than venous thrombosis, and our own observation lead to this same conclusion. In some patients with mitral valve vegetations, however, embolic occlusion of cerebral arteries may occur.
Diffuse cortical atrophy occurring in PAPS is clinically associated with dementia. Westerman reported the occlusion of small meningeal and cortical arterioles by thrombi and marked endothelial hyperplasia in PAPS patients with dementia (15). These findings suggest that diffuse cortical atrophy can be caused by multiple cortical infarcts with thromboses of small arterioles, resulting in dementia (5, 16, 17).
Dural sinus thrombosis was not a common finding in this small series. Several PAPS cases with dural sinus thrombosis have been reported (18), and this may be associated with hypercoaguable condition of the blood in cases involving PAPS. Carhuapoma reported that the presence of antiphospholipid antibodies was one of the important factors for the development of dural sinus thrombosis (19). In PAPS patients, dural sinus thrombosis may occur in a younger age group and with more extensive involvement of the venous system than other causes of venous thrombosis such as pregnancy, hyperviscosity syndrome, Behcet's disease, coagulopathy including activated protein C resistance, and other collagen vascular diseases. However, the exact mechanisms by which antiphospholipid antibodies promote thrombosis remain unknown. Focal hemorrhage in the basal ganglia was detected in two patients and this may also be caused the thromboses of small arteries or venules.
In conclusion, neuroradiologic findings of PAPS include multiple hyperintense foci in the deep white matter as seen on T2-weighted MR images, large territorial infarct, diffuse cortical atrophy, focal hemorrhage, and dural sinus thrombosis. Hypercoagulability of the blood and secondary vascular thrombosis appear to be the main pathogenetic mechanism leading to various kinds of brain lesions. Although the neuroradiologic findings are nonspecific, PAPS should be suspected when young and middle aged adults show the above mentioned CT or MR findings, and other risk factors for ischemic stroke or vascular thrombosis are not present.
Figures and Tables
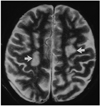 | Fig. 1A 47-year-old man who at the time of examination had suffered headache and general weakness for one month (case 4). Axial T2-weighted MR image shows multiple high signal intensities(arrows) in the white matters at the level of the centrum semiovale. |
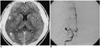 | Fig. 2A 32-year-old man who reported the onset of hemiplegia three hours prior to examination (case 2).
A. Contrast-enhanced CT scan shows subtle low density in the right temporal lobe and insular cortex(arrows), suggesting a hyper-acute infarct in the territory of the right middle cerebral artery.
B. Frontal view of right internal carotid angiogram shows complete occlusion of the right middle cerebral artery at M1 portion(arrow).
|
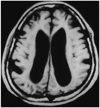 | Fig. 3A 34-year-old woman with progressive dementia (case 8). Axial T1-weighed MR image shows diffuse cerebral atrophy with prominent sulci and dilatation of the lateral ventricles. |
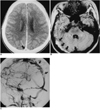 | Fig. 4A 25-year-old woman who had suffered right hemiplegia for 2 days (case 6).
A. Enhanced CT scan shows a filling defect in the superior sagittal sinus (empty delta sign, arrow).
B. FLAIR axial T2-weighted MR image shows abnormally high signal intensity in the right transverse sinus, suggesting thrombosis (arrows).
C. Oblique view of right internal carotid angiogram during the venous phase shows multiple filling defects in the superior sagittal sinus (arrows).
|
References
1. Harris EN, Gharavi AE, Hughes GRV. Antiphospholipid antibodies. Clin Rheum Dis. 1985. 11:591–609.
2. Harris EN, Baguley E, Asherson RA, et al. Clinical and serological features of the "antiphospholipid syndrome". Br J Rheumatol. 1987. 26:S. 19.
3. Hughson MD, Mccarty GA, Brumback RA. Spectrum of vascular pathology affecting patients with the antiphospholipid syndrome. Hum Pathol. 1995. 26:716–724.
4. Asherson RA, Khamashta MA, Ordi-Ros J, et al. The "primary" antiphospholiupid syndrome: major clinical and serological features. Medicine. 1989. 68:366–374.
5. Gharavi AE, Wilson WA. The syndrome of thrombosis, thrombocytopenia, and recurrent spontaneous abortions associated with antiphospholipid antibodies: Hughes syndrome. Lupus. 1996. 5:343–344.
6. Provenzale JM, Heinz ER, Ortel TL, et al. Antiphospholipid antibodies in patients without SLE: neuroradiologic findings. Radiology. 1994. 192:531–537.
7. Provenzale JM, Barboriak DP, Allen NB, Ortel TL. Patients with antiphospholipd antibodies: CT and MR findings of the brain. AJR. 1996. 167:1573–1578.
8. Toubi E, Khamashta MA, Panarra A, Hugham GRV. Association of antiphospholipid antibodies with central nervous system disease in SLE. Am J Med. 1995. 99:397–401.
9. Brey RL, Gharavi AE, Lockshin MD. Neurologic complications of antiphospholipid antibodies. Rheumatol Clin North Am. 1993. 4:833–850.
10. Oeffinger KC, Roaten SP. Antihpospholipid syndrome. J Fam Prac. 1994. 38:611–619.
11. Petri M. Pathogenesis and treatment of the antiphospholipid antibody syndrome. Med Clin North Am. 1997. 81:151–177.
12. Aron AL, Gharavi AE, Shoenfeld Y. Mechanisms of action of antiphospholipid antibodies in the antiphospholipid antibodies in the antiphospholipd syndrome. Int Arch Allergy Immunol. 1995. 106:8–12.
13. Lechner K, Pabinger-Fasching I. Lupus anticoagulants and thrombosis: a study of 25 cases and review of the literature. Haemostasis. 1985. 15:254–262.
14. Levine SR, Brey RL. Neurological aspects of antihpospholipid antibody syndrome. Lupus. 1996. 5:347–353.
15. Westerman EM, Miles JM. Neuropathologic findings in multi-infarct dementia associated with anticardiolipin antibody. Arthritis Rheum. 1992. 35:1038–1041.
16. Coull BM, Bourdette DN, Goodnight SH, Briley DP, Hart R. Multiple cerebral infarctions and dementia as associated with anticardiolipin antibodies. Stroke. 1987. 18:1107–1112.
17. Westerman EM, Miles KM, Backonja M, Sundstrom WR. Neuropahtologic findings in multi-infarct dementia associated with anticardiolipin antibody. Arthritis Rheum. 1992. 9:1038–1041.
18. Levin SR, Kieran S, Puzio K, et al. Cerebral venous thrombosis with lupus anticoagulants: report of two cases. Stroke. 1987. 18:801–804.
19. Carhuapoma JR, Mitsias P, Levine SR. Cerebral venous thrombosis and antiphospholipid antibodies. Stroke. 1997. 28:2363–2369.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download