Abstract
Objective
To evaluate the usefulness of MR imaging for diseases of the small intestine, emphasizing a comparison with CT.
Materials and Methods
Thirty-four patients who underwent both CT and MR imaging using FLASH 2D and HASTE sequences were analyzed. All patients had various small bowel diseases with variable association of peritoneal lesions. We compared the detectabilities of CT and MR imaging using different MR pulse sequences. The capability for analyzing the characteristics of small intestinal disease was also compared.
Results
MR imaging was nearly equal to CT for detecting intraluminal or peritoneal masses, lesions in the bowel and mesentery, and small bowel obstruction, but was definitely inferior for detecting omental lesions. The most successful MR imaging sequence was HASTE for demonstrating bowel wall thickening, coronal FLASH 2D for mesenteric lesions, and axial FLASH 2D for omental lesions. MR imaging yielded greater information than CT in six of 12 inflammatory bowel diseases, while it was equal to CT in six of seven neoplasms and inferior in five of seven mesenteric ischemia. In determining the primary causes of 15 intestinal obstructions, MR imaging was correct in 11 (73%) and CT in nine (60%) patients.
Despite the advantages of multiplanar imaging and sequential varieties, there has been little attempt to utilize MR imaging for diagnosing diseases of the small intestine. This is primarily due to the lack of a suitable, cost-effective oral contrast agent. In fact, some researchers have attempted conventional MR imaging for evaluation of the gastrointestinal tract (1-5). However, because of the long acquisition time, bowel marking is very poor due to motion artifact and/or peristaltic bowel movement. In contrast, recently introduced breath-hold turbo spin-echo MR imaging produces excellent bowel marking with a hyperintense lumen on T2-weighted images if the bowel is filled with an appropriate amount of fluid. Of the newer techniques, HASTE (half-Fourier acquisition single-shot turbo spin-echo) technique is a high-speed, heavily T2-weighted sequence with a great sensitivity for fluid detection (6, 7); its fast acquisition time of approximately 1 sec per slice allows good images even for uncooperative and ill patients. Their efficacy has also been proven for biliary system (8), urinary tract (9), fetus (10), and for evaluating the small bowel obstruction (11). This advance may make it possible to use breath-hold turbo spin-echo MR imaging for examination of the small intestine without specific oral contrast agent.
In addition, most of diseases of the small intestine are commonly accompanied by omental or mesenteric changes. As a first step in the application of MR imaging for various small intestinal diseases, the feasibility of MR imaging for detecting lesions in the peritoneal cavity should also be assessed. To our knowledge, however, such a study has seldom been attempted.
The purpose of this study is to evaluate the usefulness of MR imaging for patients with small intestinal diseases, emphasizing a comparison with abdominal CT.
During the recent 3 years, 76 patients underwent MR imaging for suspected small intestinal pathology. Of these patients, 34 patients who underwent abdominal CT constituted the basis of our study. All of these patients had various small bowel diseases with peritoneal pathology, i.e., 12 patients with inflammatory bowel disease, seven with mesenteric ischemia, seven with neoplasm, and seven with small bowel adhesions, and one with phytobezoar. The remaining 42 patients were excluded because 18 patients did not have small bowel pathology and 24 did not undergo abdominal CT. Of the 34 patients, the diagnoses were made by surgery in 16 patients, retrograde small bowel biopsy during colonoscopy in nine, small bowel follow-through in four, clinical follow-up in three, and visceral angiography in two. These patients consisted of nine females and 25 males whose ages ranged between 15 and 71 years (mean, 45 years).
MR imaging was obtained with a 1.5-T superconductive unit (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany) using a body phased-array coil. The MR imaging sequences used were breath-hold T1-weighted FLASH 2D images and T2-weighted HASTE images. Coronal FLASH 2D and HASTE images were obtained in all of the 34 patients, but axial FLASH 2D images were obtained in 26 and axial HASTE images in 28. For FLASH 2D images, imaging parameters included a 117/4 msec (TR/TE), 134×256 matrix, 338×450-mm FOV, a flip angle of 70° and a 7-mm slice thickness with no interslice gap in coronal images, and a 131/4 msec (TR/TE), 113×256 matrix, 263×350-mm FOV, a flip angle of 80°, and same slice thickness with no gap in axial images. For HASTE images, parameters were 4/59 msec (TR/TE), a 128×256 matrix, 338×450-mm FOV, a flip angle of 140°, and a 7-mm slice thickness with no gap in coronal images, and 4/59 (TR/TE), a 128×256 matrix, 263×350-mm FOV, a flip angle of 140°, and same thickness with no gap in axial images. We performed MR imaging with the patient in a prone position in order to reduce respiratory and bowel movement artifacts. The FOV was maximized in coronal FLASH 2D images to improve the signal to noise ratio. No interslice gap was used for 3D reformatted images when necessary. Twenty-eight of the 34 patients were scanned using a sequential multislice technique; the other six who had respiratory difficulty were imaged with single slice acquisition. MR imaging was performed in 13 patients following ingestion of 600-900 ml of water 1 hour before imaging, immediately after small bowel follow-through in six, and without ingestion of oral agent in 15; in 12 of the 15 patients with small bowel obstruction, no oral intake was attempted. No patients used antiperistaltic drugs or intravenous contrast agent. The mean total examination time which included patient preparation and image acquisition was 18 min, with a range of 5-34 min. In this study, the upper abdominal organs, such as liver, spleen, and pancreas were not fully imaged because the study was focused on imaging the small intestine.
CT scans were performed using a Somatom Plus-S (Siemens Medical Systems, Erlangen, Germany) or on a 9800 Quick System (General Electric Medical Systems, Milwaukee, WI) scanner. Scans were obtained with contiguous 8- or 10-mm intervals from the diaphragm to the symphysis pubis. Intravenous contrast material (Iopamiro 300 [iopamidol]; Bracco, Milano, Italy) was administered as a bolus followed by rapid drip infusion. About 900 ml of E-Z CAT (E-Z-Em, Westbury, NY) was given orally 1 hr before scanning in 18 of the 34 patients; the other 16 patients were not given oral contrast agent. The interval between MR imaging and abdominal CT ranged 0-8 days (mean, 4 days). Small bowel follow-through was also available in 23 of the 34 patients.
Before reviewing MR images, positive findings in the bowel, omentum, and mesentery were recorded in each of the 34 patients using the integrated information based on the surgico-pathologic results (n = 25), small bowel follow-through (n = 23), abdominal CT (n = 34), clinical follow-up (n = 3), and angiography (n = 2); abdominal CT was included in order to obtain the data regarding the changes of omentum and mesentery. In these 34 patients, there were peritoneal or intraluminal masses (size range, 3-9 cm) in six, bowel obstruction in 15, bowel wall thickening in 23, mesenteric changes in 32, and omental changes in nine. As a first step, MR imaging and CT were separately and independently interpreted by two radiologists who did not know the finding of the other modality, with regard to the presence of postive findings. The final decision was made by concensus if interpretation differed. As a second step, two radiologists compared different MR pulse sequences in detecting these abnormality; in each patient, MR images were randomly interpreted with a consensus when interpretation differed. Finally, we compared CT and MR imaging in order to determine the capability of each modality in depicting the characteristics of various small intestinal diseases; the decision whether modality was superior, equal, or inferior to another was obtained by the concensus of two radiologists.
The bowel wall was considered to be thickened when it exceeded 4 mm in a well-distended state. Bowel obstruction was defined when bowel dilatation proximal to the obstructed site was greater than 3 cm with collapsed distal small bowel loop and colon. Of the 15 patients with small intestinal obstruction, the diagnosis was confirmed at surgery in eight and on small bowel follow-through in two. In the other five patients, the diagnosis was made by CT. All of these five patients showed diffuse dilatation of the small intestinal loop with a luminal diameter greater than 3.5 cm, as well as collapsed distal small bowel, colon, or both, and therefore belonged to the group with small bowel obstruction when using the CT criteria proposed by Fukuya et al. (12) and Gazelle et al. (13).
All of the MR images were successfully obtained without image blurring or degradation. MR imaging was compared with CT for identifying the lesions in the bowel, mesentery, and omentum (Table 1). In demonstrating intraluminal or peritoneal masses (n = 6) of a minimum diameter of 3 cm, MR imaging detected all masses, but CT missed one (100% versus 83%); CT failed to detect a mass near the surgical anastomosis in one patient who underwent subtotal gastrectomy due to gastric cancer, interpreting the mass lesion as a fluid-filled bowel loop. Of the 23 patients with bowel wall thickening, findings were positive in 17 (74%) on CT and in 18 (78%) on MR imaging. CT demonstrated a double halo or target appearance in the thickened bowel wall in five patients, and a similar appearance was detected on MR imaging in six patients (Fig. 1). The thickened bowel wall in these six patients showed double layers with a hypointense outer layer and a thin hyperintense inner layer on HASTE sequences, and on FLASH 2D images only three of these patients showed a slight hyperintense outer layer and a hypointense inner layer. In detecting mesenteric changes (n = 32), both MR imaging and CT had a sensitivity of 94% and 100%, respectively. MR imaging was inferior to CT in detecting omental lesions (56% versus 100%).
The feasibility of each coronal and axial FLASH 2D and HASTE sequence in detecting the lesions is shown on Table 2. Intraluminal or peritoneal masses were demonstrated in all MR imaging sequences. In detecting the small intestinal obstruction, coronal HASTE image was the most sensitive sequence. In demonstrating bowel wall thickening, HASTE sequences were better than FLASH 2D sequences. In detecting the mesenteric lesions, coronal FLASH 2D image of the four MR sequences was most sensitive; using this sequence, small lymph nodes of less than 1 cm in diameter and miliary implants (less than 5 mm) (Fig. 2) in the mesentery were easily detected. For detecting omental changes, axial FLASH 2D sequence was most successful.
Table 3 shows the comparison of MR imaging and CT for depicting the characteristics of various small intestinal diseases. Of the 12 patients with inflammatory bowel disease, i.e. six intestinal tuberculosis, five Crohn's disease, and one salmonella enteritis, MR imaging was superior to CT in six (50%); five with Crohn's disease and one with intestinal tuberculosis. In Crohn's disease, some of the characteristic findings, i.e., bowel wall thickening along the mesenteric border due to linear ulceration, changes in the antimesenteric border, localized fibrofatty proliferation, and minimal mesenteric lymphadenopathy were better demonstrated on MR images than CT (Fig. 3). In six patients with intestinal tuberculosis (Fig. 4), MR imaging was superior to CT in one, equal in three, and inferior in two. MR images failed to demonstrate the most diagnostic findings of abdominal tuberculosis in two patients, i.e., calcification or a low-attenuated center due to caseous necrosis in the enlarged nodes.
In seven patients with neoplasm (three adenocarcinomas, two lymphomas, and two leiomyosarcomas) (Figs. 4, 5), MR imaging was equal to CT in six patients but was inferior in one with peritoneal carcinomatosis due to poor demonstration of the omental infiltration.
Of the seven patients with mesenteric ischemia (Fig. 1), MR imaging provided additional information in one patient with polyarteritis nodosa; in this patient, mesenteric changes adjacent to the ischemic segment was more clearly seen on MR imaging than CT. In another one patient, MR imaging missed small emboli within the mesenteric vessels.
In all 15 patients with small intestinal obstruction, MR imaging successfully detected the presence as well as the sites of obstruction. In determining the causes of bowel obstruction, i.e. adhesions and bands (Fig. 6) in seven, post-inflammatory stricture in four, peritoneal carcinomatosis in two, adenocarcinoma in one, and phytobezoar in one, the diagnosis was correct in 11 (73%) of the 15 patients on MR imaging and in nine (60%) on CT.
Despite the great potential of MR imaging for depicting changes in each layer of the bowel wall in vitro study (14, 15), a drawback to the clinical use of MR imaging is the absence of an optimal oral contrast agent. It is still controversial whether a negative or positive oral contrast agent is necessary for lesion detection (16-20). Since HASTE sequences are heavily T2-wighted images sensitive to fluid-filled structures, we assumed that MR examination of the small intestine might be sucessful without a specific oral contrast agent. In fact, MR imaging cannot always be performed at the appropriate time in busy clinical practice even when patient's bowel is optimally distended. Additional use of intravenous contrast infusion study may increase the usefulness of MR imaging, but we did not attempt it because it will increase the cost.
Comparison of MR imaging and CT showed that MR imaging was nearly equal to CT for detecting peritoneal or intraluminal masses, bowel wall thickening, and mesenteric changes. However, MR imaging was definitely inferior to CT for detecting omental lesions. It was difficult to detect omental lesions in the absence of ascites. Axial MR imaging was superior to coronal MR imaging for detecting such lesions because of their anatomical location. Even though further study is necessary, HASTE sequences were comparable with CT for the morphological evaluation of bowel lesions. In addition, the target or double halo appearance observed on CT were also well demonstratrated on MR imaging. However, it should be mentioned that in cases with heterogeneous bowel thickening due to the submucosal collection of hemorrhage or edematous fluid, the finding of bowel wall thickening may be missed on HASTE sequences because of similarity of signal intensity between thickened bowel wall and intraluminal fluid.
Compared to HASTE sequences, FLASH 2D images, especially with coronal plane, are valuable for detecting mesenteric changes, such as miliary implants and lymphadenopathy. For detecting omental lesions, axial FLASH 2D images are most useful. On the other hand, both coronal and axial HASTE sequences are very important for the evaluation of bowel wall changes. Coronal HASTE images give the impression of 'small bowel follow-through', if the dilated bowel is filled with fluid. Therefore, these sequences provide an easy anatomical orientation of bowel obstruction or unusual bowel course after surgery.
Most inflammatory bowel diseases exhibit thickened bowel wall, perienteric infiltration, and varying degrees of lymphadenopathy. In our study, MR imaging was definitely advantageous for those cases of Crohn's disease, because of its ability to demonstrate the well-known radiologic characteristics. Minimal lymphadenopathy commonly occurring in Crohn's disease is easily overlooked on CT due to its similar size to the adjacent vessels, but is well demonstrated on MR imaging. However, MR imaging is inferior to CT for evaluating patients with intestinal tuberculosis. Despite easy detection of miliary implants and diffuse lymphadenopathy, the most diagnostic CT findings of abdominal tuberculosis, i.e., calcification or a low-attenuated center due to caseous necrosis in the enlarged nodes, are missed on MR imaging. Although our results showed that MR imaging was more accurate than CT for analyzing the characteristics of inflammatory bowel disease, further study is needed in order to determine whether MR imaging can offer greater information than CT, since our study did not include a variety of inflammatory bowel diseases.
In evaluating primary or secondary gastrointestinal malignancy or other submucosal tumors, MR imaging seems to have nearly the same capability as CT for identifying lesions in the bowel and peritoneal cavity. Because of multiplanar imaging capability, MR imaging appears to be more effective than CT for analyzing the morphologic characteristics of the tumor. Although detection of an intraluminal mass depends largely on its size or the degree of bowel dilatation proximal to the lesion, introduction of an effective oral contrast agent will improve the sensitivity of MR imaging for detecting bowel lesions; a negative oral contrast agent is considered to be more effective than a positive agent for intraluminal mass detection, since most benign and malignant small bowel neoplasms show intermediate signal intensity on HASTE images. Some researchers stress the importance of peritoneal thickening on contrast-enhanced MR images as a finding of peritoneal tumor seeding (21). However, because we did not use intravenous contrast infusion, this finding was not analyzed.
CT is a modality of choice for patients with small bowel obstruction, but it still poses problems in some instances in revealing the site of small bowel obstruction, primarily due to inherent limitations of its imaging plane. Coronal MR images appear to be more useful than axial for searching for an obstructed site, because they produce comparable image planes as small bowel follow-through. Moreover, in equivocal cases, more detailed examination of the obstructed site can be made with the help of 3D reformatted images. As additional advantages, MR imaging can be performed immediately after small bowel follow-through. There is no need of oral contrast since the fluid in the dilated loops already provides a good contrast material. For determining the primary cause of small bowel obstruction, our study also showed MR imaging to be more or less superior to CT (73% versus 60%). However, both MR imaging and CT have difficulty in determining the causes (inflammatory vs malignant) of obstruction when a short segmental stricture is present.
In six of our seven patients with mesenteric ischemia, MR imaging did not provide more information than did CT. Since contrast enhancement pattern of the bowel wall is reported to be very important for establishing a diagnosis of mesenteric ischemia (22), additional use of intravenous contrast infusion study appears necessary in such patients. Furthermore, the most critical problem with MR imaging is poor sensitivity for tiny or small emboli or thrombi within the mesenteric vessels, as was noted in one of our patients.
We have attempted to test the efficacy of MR imaging in various small intestinal diseases. MR imaging was nearly equal to CT in many instances for demonstrating bowel wall changes as well as for detecting intraluminal or peritoneal masses or mesenteric lesions. However, additional use of intravenous contrast infusion study appears necessary, especially in patients with suspected mesenteric ischemia. Although further investigation will be required, MR imaging can be used as an alternate modality of choice for ima-ging the patients with suspected Crohn's disease and small intestinal neoplasms or obstructions.
Figures and Tables
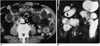 | Fig. 1Intestinal ischemia in a 74-year-old man.
A. Contrast-enhanced CT scan shows bowel wall thickening with a target sign (arrows). Note regional mesenteric haziness and vascular engorgement (arrowheads).
B. Coronal HASTE MR image shows target sign in thickened bowel wall (arrows).
|
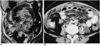 | Fig. 2Tuberculous peritonitis in a 24-year-old woman.
A. Coronal FLASH 2D MR image shows miliary implants scattered in the mesentery (M). A loculated intraperitoneal fluid collection (F) is also noted.
B. Contrast-enhanced CT scan shows better visualization of miliary lesions in the greater omentum and mesentery as well as ascites.
|
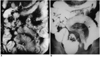 | Fig. 3A 35-year-old man with Crohn s disease.
A. Coronal HASTE MR image demonstrates eccentric bowel wall thickening along mesenteric borders (arrows) of the bowel loops. Note bowel separation due to fibrofatty proliferation (arrowheads).
B. Small bowel follow through shows marginal ulcer (arrowheads) along the mesenteric border of the small intestine. Note inflammatory stricture (arrows) of pelvic bowel loop along with regional bowel separation due to inflammatory infiltrates and fibrofatty proliferation.
|
 | Fig. 4Leiomyosarcoma of the jejunum in a 71-year-old man.
A. Contrast-enhanced CT scan shows a poorly enhanced soft tissue mass (*) in the pelvis, partially attaching to the adjacent bowel.
B. Coronal HASTE MR image shows a hyperintense soft tissue mass (straight arrows). In contrast to CT image (A), lobulated configuration is well demonstrated on this image. Intestinal loop (curved arrow) is entrapped within the mass lesion.
|
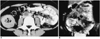 | Fig. 5Intestinal lymphoma developing intussusception in a 30-year-old woman.
A. Contrast-enhanced CT scan shows minimal bowel wall thickening (arrows) in the contrast-filled small intestinal loops as well as evidence of intussusception (*). At surgery, intussusception developed secondary to a polypoid intraluminal mass in the ileum.
B. Coronal HASTE MR image also shows intussusception (arrows) and minimal bowel wall thickening (arrowheads).
|
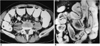 | Fig. 6Simple intestinal obstruction due to bowel adhesions in a 55-year-old man.
A. Contrast-enhanced CT scan shows end-on view of narrowed intestinal segment (arrows) just proximal to the obstructed site.
B. Coronal HASTE MR image shows diffuse dilatation of small intestinal loop with tapered narrowing at the obstructed site (curved arrow).
|
Table 1
Comparison of CT & MRI for Detecting the Positive Findings in the Bowel and Peritoneal Cavity

References
1. Chou CK, Chen LT, Sheu RS, Wang ML, Jaw TS, Liu GC. MRI manifestations of gastrointestinal wall thickening. Abdom Imaging. 1994. 19:389–394.
2. Chou CK, Chen LT, Sheu RS, et al. MRI manifestations of gastrointestinal lymphoma. Abdom Imaging. 1994. 19:495–500.
3. Ha HK, Lee EH, Lim CH, et al. Application of MRI for small intestinal diseases. J Magn Reson Imaging. 1998. 8:375–383.
4. Madsen SM, Thomsen HS, Munkholm P, Schlichting P, Davidsen B. Magnetic resonance imaging of Crohn disease: early recognition of treatment response and relapse. Abdom Imaging. 1997. 22:164–166.
5. Beers BV, Grandin C, Kartheuser A, et al. MRI of complicated anal fistulae: comparison with digital examination. J Comput Assist Tomogr. 1994. 18:87–90.
6. Regan F, Bohlman ME, Khazan R, Rodriguez R, Schultze-Haaakh H. MR urography using HASTE imaging in the assessment of ureteric obstruction. AJR. 1996. 167:1115–1120.
7. Aerts P, Hoe LV, Bosmans H, Oyen R, Marchal G, Baert AL. Breath-hold MR urography using the HASTE technique. AJR. 1996. 166:543–545.
8. Miyazaki T, Yamashita Y, Tsuchigame T, Yamamoto H, Urata J, Takahashi M. MR cholangiopancreatography using HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequences. AJR. 1996. 166:1297–1303.
9. Tang Y, Yamashita Y, Namimoto T, et al. The value of MR urography that uses HASTE sequences to reveal urinary tract disorders. AJR. 1996. 167:1497–1502.
10. Yamashita Y, Namimoto T, Abe Y, et al. MR imaging of the fetus by a HASTE sequence. AJR. 1997. 168:513–519.
11. Regan F, Beall DP, Bohlman ME, et al. Fast MR imaging and the detection of small-bowel obstruction. AJR. 1998. 170:1465–1469.
12. Fukuya T, Hawes DR, Lu CC, Chang PJ, Barloon TJ. CT diagnosis of small-bowel obstruction: efficacy in 60 patients. AJR. 1992. 158:765–769.
13. Gazelle GS, Goldberg MA, Wittenberg J, Halpern EF, Pinkney L, Mueller PR. Efficacy of CT in distinguishing small-bowel obstruction from other causes of small-bowel dilatation. AJR. 1994. 162:43–47.
14. Giovagnoni A, Misericordia M, Terilli F, Brunelli E, Contucci S, Bearzi I. MR imaging of ulcerative colitis. Abdom Imaging. 1993. 18:371–375.
15. Auh YH, Lim T-H, Lee DH, et al. In Vitro MR imaging of the resected stomach with a 4.7-T superconducting magnet. Radiology. 1994. 191:129–134.
16. Pattern RM, Moss AA, Fenton TA, Elliot S. OMR, A positive bowel contrast agent for abdominal and pelvic MR imaging: safety and imaging characteristics. J Magn Reson Imaging. 1992. 2:25–34.
17. Patten RM, Lo SK, Philips JJ, et al. Positive bowel contrast agent for MR imaging of the abdomen: phase II and III clinical trials. Radiology. 1993. 189:277–283.
18. Ros PR, Steinman RM, Torres GM, et al. The value of barium as a gastrointestinal contrast agent in MR imaging: a comparison study in normal volunteers. AJR. 1991. 157:761–767.
19. Anderson CM, Brown JJ, Balfe DM, et al. MR imaging of Crohn disease: use of perflubron as a gastrointestinal contrast agent. J Magn Reson Imaging. 1994. 4:491–496.
20. Hiraishi K, Narabayashi IN, Fujita O, et al. Blueberry juice: preliminary evaluation as an oral contrast agent in gastrointestinal MR imaging. Radiology. 1995. 194:119–123.
21. Low RN, Sigeti JS. MR imaging of peritoneal disease: comparison of contrast-enhanced fast multiplanar spoiled gradient-recalled and spin-echo imaging. AJR. 1994. 163:1131–1140.
22. Ha HK, Kim JS, Lee MS, et al. Differentiation of simple and strangulated small-bowel obstructions: usefulness of known CT criteria. Radiology. 1997. 204:507–512.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download