Abstract
Renal inflammatory pseudotumor is a very rare benign condition of unknown etiology characterized by proliferative myofibroblasts, fibroblasts, histiocytes, and plasma cells. In the case we report, the lesion appeared on contrast-enhanced power Doppler US images as a well-defined hypoechoic mass with intratumoral vascularity, and on CT as a low-attenuated mass. Differentiation from malignant renal neoplasms was not possible.
Inflammatory pseudotumor is a rare benign condition of unknown etiology characterized by proliferative myofibroblasts, fibroblasts, histiocytes, and occasionally, plasma cells and lymphocytes (1). Its histogenesis is uncertain, but the lesion is generally regarded as reactive or postinfectious, originating in an inflammatory process. It has been reported to occur in many organs, including the lung and orbit, though its occurrence in the kidney is very rare, and to our knowledge, only four such cases have been described in the literature (2, 3). None of these reports included the radiologic findings.
In what we believe to be the first such report, we describe the radiologic findings of an inflammatory pseudotumor of the kidney studied with US, retrograde pyelography (RGP) and CT.
A 60-year-old man was referred for further evaluation of painless gross hematuria, first noted ten days earlier. His past medical history and the results of physical examination were unremarkable, except for mild right flank discomfort. Urinalysis revealed numerous red blood cells (RBC>100/HPF), while other laboratory tests, including urine culture and cytology, were all negative.
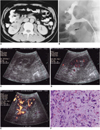
Abdominal CT performed at another hospital revealed the presence of a well-defined right renal mass involving the lower pole (Fig. 1A). The lesion showed low attenuation, and its margin with the renal parenchyma was ill defined. Neither renal vein invasion nor perirenal extension was evident. RGP revealed a dilated lower pole calyx with irregular filling defect (Fig. 1B). For US examination, real-time equipment (HDI 3000; Advanced Technology Laboratories, Bothell, WA) with a 2-4 MHz curved array transducer was used. Gray-scale US revealed a round, homogeneously hypoechoic lesion, 1.6 cm in diameter, located mainly within the renal sinus. On power Doppler US, multiple vascularities meandering around the mass were identified, but none were seen within it (Fig. 1C). To demonstrate and characterize tumor vascularity, contrast-enhanced power Doppler US was performed. The contrast agent was SH U 508A (Levovist; Schering, Berlin, Germany), prepared for injection by shaking with 11 mL of water for 5 seconds. After standing for 2 minutes for equilibration, 12 mL of a 200 mg/mL suspension was injected into the antecubital vein at 0.2 ml/sec. From 150 seconds to 7 minutes after injection, serial dynamic power Doppler US data were obtained every 30 seconds. US protocols were as follows: a pulse repetition frequency of 1,000 Hz and a medium wall (high-pass) filter for conventional power Doppler US, and a pulse repetition frequency of 700 Hz and a low wall filter for harmonic power Doppler US.
The harmonic power Doppler US images obtained 150 seconds after injection showed dot-like and linear power Doppler US signals within the mass (Fig. 1D), and the conventional power Doppler US images obtained 7 minutes after injection also showed these same signals (Fig. 1E). However, severe blooming artifacts were observed around and within the kidney.
On the basis of the clinical and radiological findings, a malignancy such as transitional cell carcinoma was suspected, and laparoscopic nephrectomy was performed. Gross examination disclosed a 1.6-cm sized round, firm, reddish mass with hemorrhage in the side of the lower pole calyx. The renal pelvis and capsule were intact, but hyperemia was noted in the pelvis. Microscopic examination of the lesion revealed marked myofibroblastic proliferation, with hemorrhage and infiltration of lymphocytes, plasma cells and macrophages. Mitotic activity was low, and cellular atypism was minimal (Fig. 1F). Immunohistochemical staining was negative for cytokeratin, myoglobin and desmin, but positive for vimentin. These findings were consistent with inflammatory myofibroblastic pseudotumor.
Lesions classified as 'inflammatory pseudotumor', 'postinflammatory', or 'plasma cell granuloma' have been found in a variety of locations: the lung, orbit, parotid, pleura, stomach, and liver. Inflammatory pseudotumor of the urogenital tract is usually located in the urinary bladder or prostate gland, and may also occur in the kidney, though this is rare; a review of the literature revealed only four such cases (2, 3). The histogenesis of inflammatory pseudotumor is uncertain, but it is generally thought to arise as the result of an inflammatory reaction to surgery, trauma, infection or malignancy. As in our patient, it may also occur without any previous insult. Histologically, inflammatory pseudotumor of the urogenital tract is typically composed of spindle cells within a collagenous or myxoid matrix, with a mixed inflammatory cell infiltrate of plasma cells, lymphocytes, and histiocytes (4). A paucity of nuclear pleomorphism and low-mitotic activity are suggestive of inflammatory pseudotumor rather than malignancy. The results of immunohistochemical staining vary, but most recent studies have suggested that the spindle cells of an inflammatory pseudotumor are of fibroblastic or myofibroblastic origin (5).
The radiologic findings of inflammatory pseudotumor in other locations have been reported in the literature but are nonspecific. US demonstrates a variable pattern of echogenicity, and the lesion has been described as hypo- or hyperechogenic with ill-defined or well-circumscribed margins (6). Enhanced CT may demonstrate homo- or heterogeneity and hypo-, iso-, or hyperdensity (7). In these pseudotumors, delayed enhancement has frequently been observed, probably because of the accumulation of extravascular contrast media in the fibrotic component within the mass (8). These variable radiologic findings may be attributed to varying degrees of fibrosis, cellular infiltration, and dynamic change occurring during the inflammatory process. In our case, CT suggested that the lesion was a well-defined mass showing low-attenuation. An area of ill-defined low-attenuation within the lower pole was histologically confirmed as combined parenchymal inflammation.
Concurrent with the development of a wide variety of US contrast agents, US techniques including power Doppler US have evolved to a point at which they show great promise in the demonstration and evaluation of tumoral vascularity. Recent studies have shown that power Doppler US used in conjunction with a contrast agent depicts more tumoral vascularity than does unenhanced power Doppler US (9). More recently, harmonic power Doppler US used with a contrast agent has provided high-quality detailed vascular information while decreasing the artifacts seen in conventional power Doppler US (10). In our case, unenhanced conventional power Doppler US showed no definite intratumoral power Doppler US signals, but contrast-enhanced power Doppler US showed an increase in the number and intensity of these. In the harmonic mode, the intensity of contrast-enhanced power Doppler US signals in the tumor was similar to that noted when the conventional mode was used, but with the harmonic mode, blooming artifacts around and within the kidney were markedly decreased. Although in-depth studies regarding the usefulness of contrast-enhanced power Doppler US in the harmonic mode have not been published, we might expect the modality to be useful for demonstrating tumor vascularity.
In our case, the preoperative radiologic diagnosis was transitional cell carcinoma (TCC). Demonstration of a filling defect by intravenous urography (IVU) or RGP is generally accurate and is the historic standard for diagnosis of upper urinary tract and ureteral tumors. The differential diagnosis of a radiolucent filling defect in the intrarenal collecting system or renal pelvis may include transitional cell carcinoma, blood clot and radiolucent calculus. In the diagnosis of this last-mentioned condition, US-because of the echogenicity and acoustic shadowing it characteristically demonstrates-can be very helpful. The appearance of TCC on conventional US images appears to be nonspecific, however, and differentiation from blood clots or other filling defects may be troublesome. In our case, contrast-enhanced power Doppler US clearly demonstrated intratumoral vascularity, which was not depicted on conventional power Doppler US, and the modality was helpful for differentiation from blood clots.
At all sites, inflammatory pseudotumors clinically masquerade as malignant growths, and their clinical significance thus lies in the difficulty encountered in preoperatively excluding malignancy. As in cases involving inflammatory pseudotumor at other locations, the clinical and radiological features of this renal mass mandated surgical removal: malignant disease could not be reliably excluded.
In summary, we report a case of inflammatory pseudotumor, a very rare benign renal mass. The lesion was depicted on contrast-enhanced power Doppler US as a well-defined hypoechoic mass with intratumoral vascularity, and on CT as a low-attenuated mass. Differentiation from malignant renal neoplasm was not possible.
Figures and Tables
Fig. 1
A 60-year-old man with gross hematuria first noted ten days earlier.
A. Delayed enhanced CT image shows a well-circumscribed, right renal mass (arrow) involving the lower pole calyx. Areas of low attenuation (arrowheads) are present in adjacent renal parenchyma.
B. Retrograde pyelography revealed a dilated lower pole calyx with irregular filling defect (arrow).
C. Gray-scale US demonstrated a round, homogeneously hypoechoic lesion, 1.6 cm in diameter, at the lower pole of the right kidney (not shown). Unenhanced power Doppler US revealed no definite tumoral vascularity.
D. Harmonic power Doppler US image obtained 150 seconds after the injection of contrast agent shows dot-like, linear power Doppler US signals within the mass (arrow). These were not depicted by unenhanced power Doppler US (C).
E. Conventional power Doppler US image obtained 7 minutes after injection also shows dot-like, linear power Doppler US signals. Compared with harmonic power Doppler US, severe blooming artifacts around and within the kidney are noted (curved arrows).
F. Photomicrograph of a histologic specimen shows marked proliferation of myofibroblasts and capillaries with infiltration of lymphocytes, plasma cells and macrophages. Mitotic activity was low, and cellular atypism was minimal.
References
1. Horiuchi R, Uchida T, Kojima T, et al. Inflammatory pseudotumor of the liver: Clinicopathologic study and review of the literature. Cancer. 1990. 65:1583–1590.
2. Bell ND, Gavras JN, Donnell CA, Rodning CB. Renal inflammatory pseudotumor. South Med J. 1998. 91:1050–1053.
3. Vujanic GM, Berry PJ, Frank JD. Inflammatory pseudotumor of the kidney with extensive metaplastic bone. Pediatr Pathol. 1992. 12:557–561.
4. Young RH, Scully RE. Pseudosarcomatous lesions of the urinary bladder, prostate gland, and urethra: A report of three cases and review of the literature. Arch Pathol Lab Med. 1987. 111:354–358.
5. Jones EC, Clement PB, Young RH. Inflammatory pseudotumor of the urinary bladder: A clinicopathological, immunohistochemical, ultrastructural, and flow cytometric study of 13 cases. Am J Surg Pathol. 1993. 17:264–274.
6. Materne R, Van Beers BE, Gigot JF, et al. Inflammatory pseudotumor of the liver: MRI with mangafodipir trisodium. J Comput Assist Tomogr. 1998. 22:82–84.
7. Abehsera M, Vilgrain V, Belghiti J, Flejou JF, Nahum H. Inflammatory pseudotumor of the liver: radiologic-pathologic correlation. J Comput Assist Tomogr. 1995. 19:80–83.
8. Nam KJ, Kang HK, Lim JH. Inflammatory pseudotumor of the liver: CT and sonographic findings. AJR. 1996. 167:485–487.
9. Kim AY, Kim SH, Kim YJ, et al. Contrast-enhanced power Doppler sonography for the differentiation of cystic renal lesions: preliminary study. J Ultrasound Med. 1999. 18:581–588.
10. Choi BI, Kim TK, Han JK, et al. Vascularity of hepatocellular carcinoma: assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology. 2000. 214:381–386.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download