Abstract
Malignant transformation of teratoma in the anterior mediastinum is rare; the mass usually has a long history and is seen in older patients. We report a case of teratoma with malignant transformation in the anterior mediastinum, complicated by rupture. CT revealed a lobulated, inhomogeneous cystic mass with a fat component and wall calcifications. The lateral wall was disrupted and consolidation in the adjacent left upper lobe was noted, suggesting rupture. A heterogeneously enhanced solid portion, obliterating the fat plane between the mass and the great vessels was present in the medial aspect of the mass, and pathologic examination demonstrated the presence of adenocarcinoma.
Pathologically, malignant teratoma can be divided into three types: immature teratoma; teratoma with other malignant germ cell tumor components such as yolk sac tumor, embryonal carcinoma, choriocarcinoma, and seminoma; and teratoma with malignant transformation (TMT) (1, 2). TMT is a non-germ cell malignant tumor arising from a pre-existing mature teratoma, and in the mediastinum is extremely rare (3). Mediastinal teratoma occasionally ruptures, and if it occurs, serious complications result (4). We describe a case of adenocarcinoma arising from a mature teratoma in the anterior mediastinum, complicated by rupture.
A 49-year-old man in whom a mediastinal mass had been present for twenty years was admitted to hospital, complaining chiefly of hemoptysis. Surgery had been recommended, but he had refused this option, denying up to the time of hospitalization any symptoms related to this lesion.
Chest radiograph revealed a 10×12 cm mass in the anterior mediastinum, as well as ill-defined consolidation in the left upper lobe. Contrast-enhanced CT scanning demonstrated a lobulated, inhomogeneous cystic mass with a fat component (HU = -45) and calcified wall in the anterior mediastinum. The lateral wall of the mass was focally disrupted and consolidation with the fat component in the adjacent left upper lobe was noted, thus suggesting that the tumor had ruptured (Fig. 1A). An inhomogeneously enhanced solid portion was noted in the medial aspect of the mass and extended to the upper mediastinum, obliterating the fat plane between the mass and the mediastinal vessels (Fig. 1B).
Thoracotomy demonstrated that the mass was rubbery, with hard components, and invaded the right brachiocephalic artery, the innominate vein, the ascending aorta, and the pericardium. The mass had ruptured into the left upper lobe, but due to extensive invasion by vessels, was incompletely resected. Decompression and plication of the left upper lobe was performed, and pathologic examination showed that the excised mass was a 10×8 cm cystic tumor with a rugged, pale brown external surface. It contained yellowish brown material with a few hair like structures, and cut section revealed a round, pale gray solid area in the wall that was firmly attached to the mediastinal structures. Microscopically, while most of the mass was mature teratoma with hemorrhage and necrosis (Fig. 1C), the solid portion of the wall was found to be poorly differentiated adenocarcinoma (Fig. 1D). The final histologic diagnosis was poorly differentiated adenocarcinoma arising from a mature teratoma. One month after surgery, the tumor recurred at the resected site, despite chemotherapy and subsequently metastasized. Six months after surgery, the patient died.
Although teratoma is commonly found both in gonadal organs and at extragonadal sites such as the mediastinum, sacrococcygeum and pineal region, TMT is rarely found in any organ. According to the literature, malignant transformation occurs in 1-2% of ovarian dermoid cysts examined (5, 6), though little is known about the general incidence and pathologic features of TMT in the mediastinum. Most malignant transformations of mediastinal teratomas have occurred, subsequent to chemotherapy or irradiation in young patients initially presenting with a malignant germ-cell tumor (1, 2). Naturally occurring TMT has rarely been reported. Morinaga et al. (3) described a surgical case of mediastinal teratoma with poorly differentiated adenocarcinoma, and two surgical cases of TMT were observed in a study by Knapp et al. (7). Characteristically, naturally occurring TMT is seen more frequently in older patients, with a peak incidence in the fifth and sixth decades of life. Its histologic composition is exclusively carcinomatous, though its etiology remains unclear. Most reported cases had long histories of tumor, as in our case. Secondary, probably multiple, genetic events may have elicited malignant transformation of a benign teratoma among patients in whom this had been present for a long period (3). TMT is usually very aggressive and as a result of local spread, metastasis, or both, is fatal within a few months of initial diagnosis (3, 8).
CT demonstrates that mediastinal mature teratoma typically manifests as a heterogeneous, sharply marginated, spherical or lobulated anterior mediastinal mass with cystic components (8). To our knowledge, the imaging features of mediastinal TMT have never been discussed in the literature. In Morinaga's report (3), the solid papillary portions have also seen within a cystic mass were malignant foci. Chadha et al (5) and Curling et al (6) have also reported that malignant ovarian TMT was found in a solid area in the wall of a mature teratoma. In our case, the malignant focus was also found in the solid portion of the wall of the cystic mature teratoma. The medial wall of the tumor abutting the great vessel was indistinct and thick in our case, whereas in 91% of cases, a benign mature teratoma has been shown to have a sharp margin and thin wall (8). If an invading solid portion with an indistinct margin is present in the wall of a mature teratoma, the possibility of TMT should therefore be considered.
The incidence of rupture of a benign teratoma is as high as 36% (4), and several explanations as to why this tends to occur have been suggested. These include autolysis, chemical inflammation, ischemia, pressure necrosis, and infection. We speculate that since pathologic examination revealed both hemorrhage and necrosis in the mass, the most probable cause of rupture of a TMT is ischemia. Rapid growth of the malignant portion of a TMT can result in ischemia, necrosis, and rupture of the teratoma; the CT features of this latter are inhomogeneity of the internal components and changes in adjacent lung parenchyma, pleura, or pericardium, as in our case (9).
In summary, we report a case of teratoma with malignant transformation in the anterior mediastinum, complicated by rupture. A lobulated, inhomogeneous cystic mass was present, with a fat component and wall calcifications. The wall of the mass had been disrupted, and consolidation with the fat component in adjacent lung was observed, indicating rupture. A heterogeneously enhanced solid portion was noted in the medial aspect of the mass; this obliterated the fat plane between the mass and the great vessels, and was proven by pathologic examination to be adenocarcinoma.
Figures and Tables
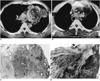 | Fig. 1A-49-year-old man and teratoma and malignant transformation.
A. Contrast-enhanced CT scan indicates the presence in the mediastinum of a lobulated low attenuated mass containing a fat component (HU =-45) (white arrows) and wall calcifications. The lateral wall of the mass is focally disrupted and consolidation with the fat component in the adjacent left upper lobe is noted (arrowheads). A heterogeneously enhanced solid portion is observed in the medial aspect of the mass, and this obliterates the fat plane between the mass and the main pulmonary artery (black arrows).
B. CT scan obtained at the origin of the great vessels shows an inhomogeneously enhanced solid portion which invades the mediastinal great vessels (white arrows) and was pathologically proven to be adenocarcinoma (★right bracheocephalic artery, ✱left common carotid artery, *left subclavian artery).
C. Light microscopic examination demonstrates that part of the resected mass is composed of mature fat (F), chrondroid cartilage (C), and glandular tissue (arrows) (×20, Hemtoxyline-eosin stain).
D. Light microscopic examination demonstrates the medial part of the mass, in which poorly differentiated adenocarcinoma crosses the fibrous outer wall (W) (×20, Hematoxyline-eosin stain).
|
References
1. Ulbright TM, Loehrer PJ, Roth LM, Einhorn LH, Williams SD, Clark SA. The development of non-germ cell malignancies within germ cell tumors. A clinicopathologic study of 11 cases. Cancer. 1984. 54:1824–1833.
2. Ahmed T, Bosl GJ, Hajdu SI. Teratoma with malignant transformation in germ cell tumors in men. Cancer. 1985. 56:860–863.
3. Morinaga S, Nomori H, Kobayashi R, Atsumi Y. Well-differentiated adenocarcinoma arising from mature cystic teratoma of the mediastinum (teratoma with malignant transformation): Report of a surgical case. Am J Clin Pathol. 1994. 101:531–534.
4. Sasaka K, Kurihara Y, Nakajima Y, et al. Spontaneous rupture: a complication of benign mature teratomas of the mediastinum. AJR. 1998. 170:323–328.
5. Chadha S, Schaberg A. Malignant transformation in benign cystic teratomas: dermoids of the ovary. Eur J Obstet Gynecol Reprod Biol. 1988. 19:329–338.
6. Curling OM, Potsides PN, Hudson CN. Malignant change in benign cystic teratoma of the ovary. Br J Obstet Gynecol. 1979. 86:399–402.
7. Knapp RH, Hurt RD, Payne WS, et al. Malignant germ cell tumors of the mediastinum. J Thorac Cardiovasc Surg. 1985. 89:82–89.
8. Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR. 1997. 169:985–990.
9. Choi S, Lee JS, Song KS, Lim T. Mediastinal teratoma: CT differentiation of ruptured and unruptured tumors. AJR. 1998. 171:591–594.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download