Abstract
Objective
Multilocular cystic renal cell carcinoma (MCRCC) is a recently described variety of renal cell carcinoma with characteristic pathologic and clinical features. The purpose of this study was to analyze the imaging findings of MCRCCs.
Materials and Methods
Ten adult patients with pathologically proven unilateral MCRCC who underwent renal US and CT were included in this study. The radiologic findings were retrospectively evaluated for cystic content, wall, septum, nodularity, calcification and solid portion by three radiologists who established a consensus. Imaging and postnephrectomy pathologic findings were compared.
Results
All patients were adults (six males and four females) and their ages ranged from 33 to 68 years (mean, 46). On US and CT images, all tumors appeared as well-defined multilocular cystic masses composed of serous or complicated fluid. In all patients, unenhanced CT scans revealed hypodense cystic portions, and in four tumors, due to the presence of hemorrhage or gelatinous fluid, some hyperdense areas were also noted. In no tumor was an expansile solid nodule seen in the thin septa, and in only one was there dystrophic calcification in a septum. Small areas of solid portion constituting less than 10% of the entire lesion were found in six of the ten tumors, and these areas were slightly enhanced on enhanced CT scans. In all patients, imaging and pathologic findings correlated closely.
Conclusion
On US and CT images, MCRCC appeared as a well-defined multilocular cystic mass with serous, proteinaceous or hemorrhagic fluid, with no expansile solid nodules in the thin septa, and sometimes with small slightly enhanced solid areas. Where radiologic examinations demonstrate a cystic renal mass of this kind in adult males, MCRCC should be included in the differential diagnosis.
Approximately 5-10% of all cases of renal cell carcinoma (RCC) present as a mainly fluid-filled cystic mass with or without solid portions (1, 2). Four basic pathologic mechanisms which lead to cystic RCC have been demonstrated (3, 4). Multilocular cystic renal cell carcinoma (MCRCC) has recently been considered a distinct subtype of cystic RCC, with characteristic gross and microscopic features (5, 6). Murad et al. (5) described MCRCC as a well-demarcated, multicystic, low-grade variant of RCC with grade 1 nuclear atypia and a possible solid portion of less than 10% of the entire lesion, which, if treated early, might be permanently cured and show good a prognosis. Eble et al. (6) suggested three diagnostic criteria of MCRCC: 1) an expansile mass is surrounded by a fibrous wall; 2) the interior of the tumor is entirely composed of cysts and septa, with no expansile solid nodule; and 3) the septa contain aggregates of epithelial cells with clear cytoplasm. Radiologic reports describing the imaging features of MCRCC have, to date, been extremely limited, however (3, 7, 8).
The purpose of this study was to analyze the radiologic findings of pathologically proven MCRCCs, and correlate these with their pathologic findings.
During the past ten years, ten cases of pathologically proven MCRCC after nephrectomy were retrospectively identified at our hospital and at others affiliated to it. All patients were adults (six males and four females), and their ages ranged from 33 to 68 years (mean, 46).
Using an Ultramark 9 HDI (Advanced Technology Laboratories, Bothell, Wash) with a 4-7 MHz convex probe, a Diasonic DRF 400 (Diasonic, Milpitas, CA ) with a 3.5-MHz probe, an Aloka SSD 650 (Aloka, Tokyo) with a 3.5 or 5 MHz probe, or an Acuson 128 (Acuson, Mountainview, CA) with a 3.5-MHz probe, US was performed in six patients. Using a GE 8800, High Speed Advantage, CTi Standard (General Electric Medical Systems, Milwaukee, WI) or a Somatom Plus VD30 (Siemens Medical Systems, Erlangen), CT images were obtained for all patients. Unenhanced CT scanning with 10-mm section thickness was followed by a bolus injection of Ultravist 300 (Iopromide 0.6234 g/mL, 140-150 cc, Shering, Berlin), and using thin sections with 5- or 7-mm collimation, enhanced CT scans were obtained during the tubular nephrographic phase.
Radiologic findings of MCRCCs in the ten patients were retrospectively evaluated in terms of site, size, nature of fluid, wall, septum, nodularity, calcification, solid portion, and contrast enhancement. The volume ratio of the solid portion and the entire lesion was roughly calculated using the following formula: (length × width × depth of the solid portion) / (length × width × depth of the entire lesion) × 100. Imaging findings were assessed by three experienced genitourinary radiologists, who established a consensus. The radiologic findings were compared with the pathologic findings of post-nephrectomy specimens. In order to rule out the possibility of recurrence or metastasis of MCRCC, patients underwent follow-up by means of US or scintigraphy.
All tumors were unilateral; the greatest diameter ranged from 3 to 12 cm (mean 5.8). The radiologic and clinical findings of these MCRCC are summarized in Table 1.
In six patients who underwent US, all tumors appeared as well-defined multilocular cystic masses without any expansile solid nodules in the thin septa (Figs. 1-3). Fluid in the multiple locules of MCRCC was anechoic in six cases, but in two, hyperechoic debris was also contained in some locules (Figs. 2, 3).
CT showed a well-defined multilocular cystic mass composed of serous and/or complicated fluid, and in no case were expansile solid nodules found in the thin septa (Figs. 1-3). Unenhanced CT revealed the cystic portions as hypodense in ten tumors (Figs. 1, 3), but in three, hyperdense areas were admixed in some locules, suggesting the presence of hemorrhage (Fig. 3) or gelatinous fluid. Small areas of solid portion constituting less than ten percent of the entire lesion were notified in six cases, and on enhanced CT scans those solid portions were slightly enhanced (Fig. 3). The mean increase in CT attenuation in the these cases was 20±8 (mean±SD) Hounsfield units during the delayed phase. Dystrophic calcification within a septum was found in one tumor (Fig. 1).
Pathologic examination revealed that all tumors were well-defined MCRCCs consisting of variably sized multiple cysts and thin septa of less than several millimeters in thickness. The cystic cavity was filled with hemorrhage in two tumors (Figs. 2, 3) and with gelatinous fluid in a further two (Fig. 1). The septa consisted of fibrous tissue, which was often collagenous. Macroscopic expansile nodules were not found in the septa of any tumors, but calcification within a septum was found in one. In six cases, yellowish solid components, characteristic of clear cell carcinoma, were limited to small areas constituting less than 10% of the entire lesion. Microscopically, the cyst walls of the tumors were lined by thin layers of clear cells in nine cases and by granular cells in one. Grade 1 nuclear atypia were present in nine cases, and a focus of grade 2 nuclear change was found in one.
During the follow-up period of 0.5-10 years (mean, 2.8), no patient showed clinical or radiological evidence of recurrent or metastatic MCRCC.
Cystic RCC includes any malignant neoplasm of renal tubular epithelium which presents as a fluid-filled mass (3). The radiographic and pathologic findings of cystic RCC are often more confusing and less specific than the findings of RCCs which are predominantly solid. Hartman et al. (3) described four basic pathologic mechanisms which lead to cystic RCC: 1) intrinsic multiloculated growth (33%); 2) intrinsic unilocular growth (cystadenocarcinoma) (31%); 3) cystic necrosis (6%); and 4) origin in the epithelial lining of a preexisting simple cyst (6%). They also classified three basic radiologic patterns of cystic RCC: unilocular cystic mass, multiloculated cystic mass, and discrete mural nodule in a cystic mass. Levy et al. (9) provided an updated pathologic-radiologic classification of cystic renal tumors and assessed diagnostic imaging capabilities. Eighty-seven cases of cystic renal tumors explored by means of multimodal imaging (US, CT, MR, arteriography) were reported, with histopathologic correlation. In their study, the most common cystic RCCs were MCRCC (33%) and Bosniak category IV pseudocystic necrotic carcinoma (31%), while less common cystic RCCs were unilocular cystic RCC (6%) and renal cyst wall carcinoma (6%). In our study, cases of MCRCC comprised 20% of cystic RCCs and 4% of RCCs presenting over a ten-year period. Bielsa et al. (10) noted that because cystic RCC was usually identified at earlier stages, had a slower growth rate, and was therefore associated with a better prognosis and longer survival than conventional RCC, a conservative surgical approach should be the treatment of choice whenever technically feasible. Ooi et al. (11) reported the high percentage of stage-1 disease (72.7%, 8/11) in cystic RCCs.
MCRCC appears to be a distinct subtype of RCC, with characteristic gross and microscopic features (5, 6). In 1957, Robinson described the first case of so-called MCRCC containing epithelial clear cells (12). In 1991, Murad et al. (5) found only six cases of MCRCC among 263 renal cysts and neoplasms during a ten-year period in which follow-up was for a minimum of two years. Neither recurrence nor metastasis was observed in any of these cases. Histologically, MCRCCs were well-demarcated multicystic lesions containing variably sized aggregates of neoplastic clear cells showing grade 1 nuclear features and little or no mitotic activity. The cystic mass was filled with gelatinous and hemorrhagic fluid. A variegated, yellowish, solid component, characteristic of clear cell carcinoma, was limited to small areas constituting less than 10% of the entire lesion. The cyst walls were densely fibrotic (12), and the lining was often devoid of epithelium. Murad et al. (5), believed that MCRCC was a low-grade variant of RCC, which, if treated early, might have a permanent cure and good prognosis. Hartman et al. (3) estimated that MCRCCs comprised 6% of RCCs. In 1998, Eble et al. (6) suggested the following diagnostic criteria for MCRCC, a neoplasm with an intrinsically cystic growth pattern, and no, or at most little, malignant potential: 1) an expansile mass is surrounded by a fibrous wall; 2) the interior of a tumor is composed entirely of cysts and septa, with no expansile solid nodule; and 3) the septa contain aggregates of epithelial cells with clear cytoplasm. The pathologic criterion that there should be no grossly visible expansile solid nodule expanding the septa, is easier to apply and more clearly distinguishes MCRCC from conventional clear-cell RCC with extensive cystic change (6). Yamashita et al. (7) described the radiologic findings of 13 MCRCCs presenting as solid masses. Although, in their cases, pathologic examination revealed multiple cysts within the tumors, smaller MCRCCs appeared solid, and radiologic examination showed that contrast enhancement or neovascularity was very slight. In our study, one small MCRCC with a largest diameter of 3 cm did not appear solid on either US or CT image. Weiss et al. (13) insisted that when MCRCC was suspected preoperatively and confirmed intraoperatively, nephron-sparing surgery should be considered. Since the nuclear grade of MCRCC is usually low (grade 1 or 2) and for a patient with MCRCC the prognosis is good, their claim is plausible.
All renal masses in our study met the diagnostic and pathologic criteria of Eble et al. (6); the radiologic features of these MCRCCs correlated closely with their gross pathologic findings. All were well-demarcated, and the interior of the tumors was entirely composed of cysts and septa, with no expansile solid nodule. The radiologic findings of thin septa without solid nodules and large locules in renal masses may be found not only in MCRCC but also in multilocular cystic nephroma (MLCN). MCRCC is reported to be predominant in adult males, with a male:female predominance of approximately 3:1 (6). Calcification may be present in the septa or pseudocapsule of more than 20% of MCRCCs (3), and in our study, septal calcification was found in one tumor. In their definition of MCRCC, Murad et al. (5) included tumors with solid masses comprising less than 10% of their total volume, and in our study, six MCRCCs (60%) had small solid areas constituting less than 10% of the entire lesion. The locules of MCRCC often contain variable amounts of new and old blood, as well as gelatinous, mucinous or proteinaceous fluid (3, 5, 7), and three MCRCCs in our study (30%) contained blood and/or gelatin. In contrast with MCRCCs, MLCNs exhibit a biphasic age and sex distribution: they occur chiefly in males aged less than four and in women aged 40 to 60. MCRCC is an obviously malignant low-grade tumor, but MLCN is almost always benign, metastasis being extremely uncommon (14). In the rare case of a malignant MLCN, the cystic spaces are often small and the stromal component is sarcomatous (3). MLCN also tends to protrude or herniate into the renal pelvis (14). The above demographic, pathologic and imaging findings may, therefore, help differentiate MCRCC from MLCN.
In conclusion, the US and CT images obtained in our study showed that MCRCC appeared as a well-defined multilocular cystic mass filled with serous, proteinaceous or hemorrhagic fluid, with no expansile solid nodules in the thin septa, but possibly with small, slightly enhanced solid areas constituting less than 10% of the entire lesion. When radiologic examinations reveal a cystic renal mass of this kind in adult males, MCRCC should be included in the differential diagnosis.
Figures and Tables
Fig. 1
A 41-year-old female with MCRCC in the left kidney (Case 2).
A. Unenhanced CT scan shows a lobulated hypodense mass (solid arrows) in the antero-lateral aspect of the left kidney. Note a hyperdense spot (open arrow) in the mass, which pathologic examination showed to be an area of calcification.
B. Enhanced CT scan shows a lobulated multiloculated cystic mass with multiple enhanced thin septa, nonenhanced fluid, and clear margin (arrows). The septa do not appear to have expanding nodules.
C. Photograph of resected specimen reveals that the tumor is well-defined and divided into multiple locules by thin septa of less than one millimeter (arrows). The locules are filled with serous and gelatinous fluid, and the septa have no expanding solid nodules. Microscopic examination (not shown here) demonstrated that the septa consist of fibrous tissue with some collagenous portions, and the cyst walls are lined by a single layer of clear epithelial cells with grade 1 nuclear atypia.
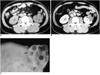
Fig. 2
A 38-year-old male with MCRCC in the right kidney (Case 5).
A. Longitudinal US image shows a well-defined multilocular cystic mass in the right kidney separated by multiple echogenic thin septa. Note associated echogenic debris in the dependent portion of the locules, suggesting blood clots (arrows).
B. Unenhanced CT scan shows a large well-defined multilocular cystic mass in the subcapsular portion of the right kidney, compressing the residual renal parenchyme medially and centrally. The variably sized cystic portions contain hypo-, iso-, or hyperdense fluid, depending on the presence of hemorrhage and/or proteinaceous fluid.
C. Enhanced CT shows a multiloculated cystic mass with enhanced thin septa, nonenhanced fluid of varying density, and some enhanced solid portions (arrows). The thin septa do not show expanding nodules.
D. Photograph of gross specimen reveals that the large subcapsular multiloculated cystic mass is composed of multiple thin septa without expanding solid nodules, a large amount of subcapsular hematoma and hemorrhagic fluid in the multicystic lesions, and a small number of solid portions constituting less than 10% of the entire lesion. Microscopic examination (not shown here) indicates that the cystic wall of the mass has extensive fibrosis, and aggregates of papillary clear cell carcinoma cells with abundant clear to eosinophilic granular cytoplasm.
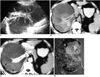
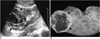
Fig. 3
A 43-year-old female with MCRCC in the right kidney (Case 6).
A. Longitudinal US scan shows a well-defined multiloculated exophytic cystic mass with multiple thin septa in the upper pole of the right kidney (solid arrows). Note the associated echogenic debris in the dependent portions of the locules, suggesting blood clots (open arrows).
B. Photograph of resected specimen reveals that the tumor is well encapsulated and divided into multiple locules by thin septa without expanding nodules (arrows). The cystic mass contains serous and hemorrhagic fluid.
References
1. Hartman DS, editor. The troublesome cystic renal mass. Proceedings of Uroradiology in Santa Fe' 97. 1997. 29–32.
2. Murphy JB, Marshall FF. Renal cyst versus tumor: a continuing dilemma. J Urol. 1980. 123:566–569.
3. Hartman DS, Davis CJ Jr, Johns T, Goldman SM. Cystic renal cell carcinoma. Urology. 1986. 283:145–153.
4. Levy P, Helenon O, Merran S, et al. Cystic tumors of the kidney in adults: radiopathologic correlations. J Radiol. 1996. 80:121–133.
5. Murad T, Komaiko W, Oyasu R, Bauer K. Multilocular cystic renal cell carcinoma. Am J Clin Pathol. 1991. 95:633–637.
6. Eble JN, Bonsib SM. Extensively cystic renal neoplasms: cystic nephroma, cystic partially differentiated nephroblastoma, multilocular cystic renal cell carcinoma and cystic hamartoma of renal pelvis. Semin Diagn Radiol. 1998. 15:2–20.
7. Yamashita Y, Miyazaki T, Ishii A, Watanabe O, Takahashi M. Multilocular cystic renal cell carcinoma presenting as a solid mass: radiologic evaluation. Abdom Imaging. 1995. 20:164–168.
8. Feldeberg MAM, van Waes PFGM. Multilocular cystic renal cell carcinoma. AJR. 1982. 138:953–955.
9. Levy P, Helenon O, Merran S, et al. Cystic tumors of the kidney in adults: radiohistopathologic correlations. J Radiol. 1999. 80:121–133.
10. Bielsa O, Lloreta J, Gelabert-Mas A. Cystic renal cell carcinoma: pathological features, survival and implications for treatment. Br J Urol. 1998. 82:16–20.
11. Ooi GC, Sagar G, Lynch D, Arkell DG, Ryan PG. Cystic renal cell carcinoma: radiological features and clinico-pathological correlation. Clin Radiol. 1996. 51:791–796.
12. Robinson GL. Perlman's tumor of the kidney. Br J Surg. 1957. 44:620–623.
13. Weiss SG 2nd, Hafez RG, Uehling DT. Multilocular cystic renal cell carcinoma: implications for nephron sparing surgery. Urology. 1998. 51:635–637.
14. Madewell JE, Goldman SM, Davis CJ Jr, Hartman DS, Feigin DS, Lichtenstein JE. Multilocular cystic nephroma: a radiographic-pathologic correlation of 58 patients. Radiology. 1983. 146:309–321.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download