INTRODUCTION
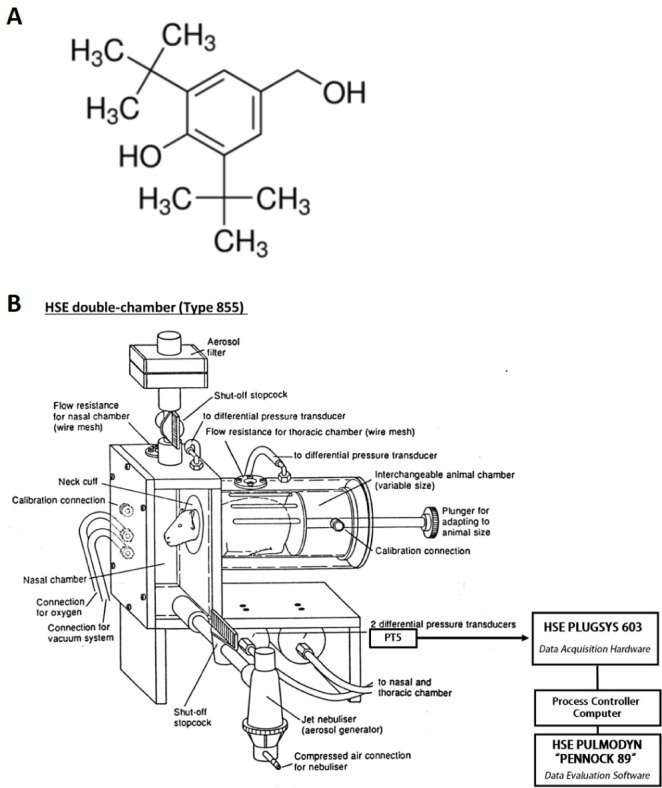 | Fig. 1Chemical structure of 2,6-di-tert-bytyl-4-hydroxymethylphenol (A) and diagram of the double-chambered plethysmograph (B) used in this experiment.This double-chambered plethysmograph is a basic method for measurement of the specific airway resistance (sRaw) together with the standard parameters tidal volume and respiratory rate in conscious animals placed into the plethysmograph box (HSE type 855, Hugo Sachs Elektronik, Germany). The phase shift between two the nasal and thoracic respiratory flows is measured with two differential pressure transducers (PT5, Grass Instument Co., USA). The PULMODYN “PENNOCK 89” software is able to record the signals and calculate the sRaw with a respiratory analyzer (7E polygraph, Grass Instument Co., USA), and the PLUGSYS 603 system is used to control the valves from the plethysmograph boxes and interface the boxes to the computer (This diagram modified from an original kindly provided by Hugo Sachs Elektronik company, Germany).
|
METHODS
Materials
Animals
Sensitization and aerosolized-OVA challenge
Measurement of airway function
Bronchoalveolar lavage and cellular analysis
Biochemical analysis in BALF
Histamine assay
Phospholipase A2 assay
Protein assay
Histopathological analysis of lung
Statistical analysis
RESULTS
Effect of DBHP on airway hyperreactivity in aerosolized OVA-induced asthmatic responses
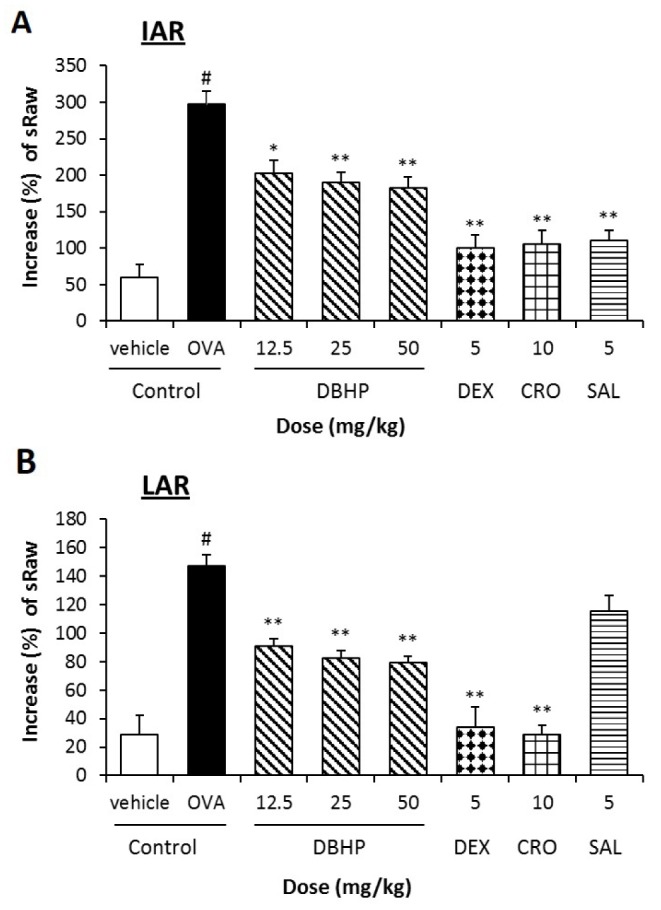 | Fig. 2Effect of DBHP on specific airway resistance (sRaw) to the antigen challenge in IAR (A) and LAR (B).Test drugs were orally administered 1 h prior to OVA challenge, additionally 12 h later. Percentage increase of sRaw (%)=[(sRaw after OVA challenge/sRaw before OVA challenge)−1]×100. Each value represents the mean±SEM (n=6). Values are statistically significant at #p<0.05 compared with the vehicle control; *p<0.05 and **p<0.01 compared with OVA control. DBHP, 2,6-di-tert-butyl-4-hydroxymethylphenol; DEX, dexamethasone; CRO, cromoglycate; SAL, salbutamol.
|
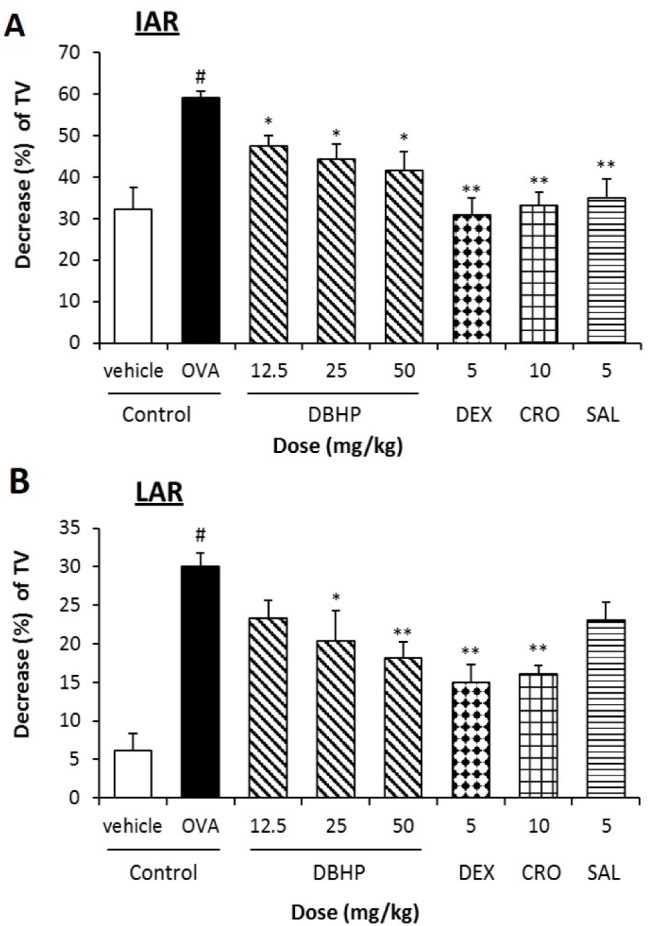 | Fig. 3Effect of DBHP on the tidal volume (TV) to the antigen challenge in IAR (A) and LAR (B).Test drugs were orally administered 1 h prior to OVA challenge, additionally 12 h later. Percentage decrease of TV (%)=[1−(TV after OVA challenge/TV before OVA challenge)]×100. Each value represents the mean±SEM (n=6). Values are statistically significant at #p<0.05 compared with the vehicle control; *p<0.05 and **p<0.01 compared with OVA control. DBHP, 2,6-di-tert-butyl-4-hydroxymethylphenol; DEX, dexamethasone; CRO, cromoglycate; SAL, salbutamol.
|
Effect of DBHP on the recruitment of leukocytes in BALF
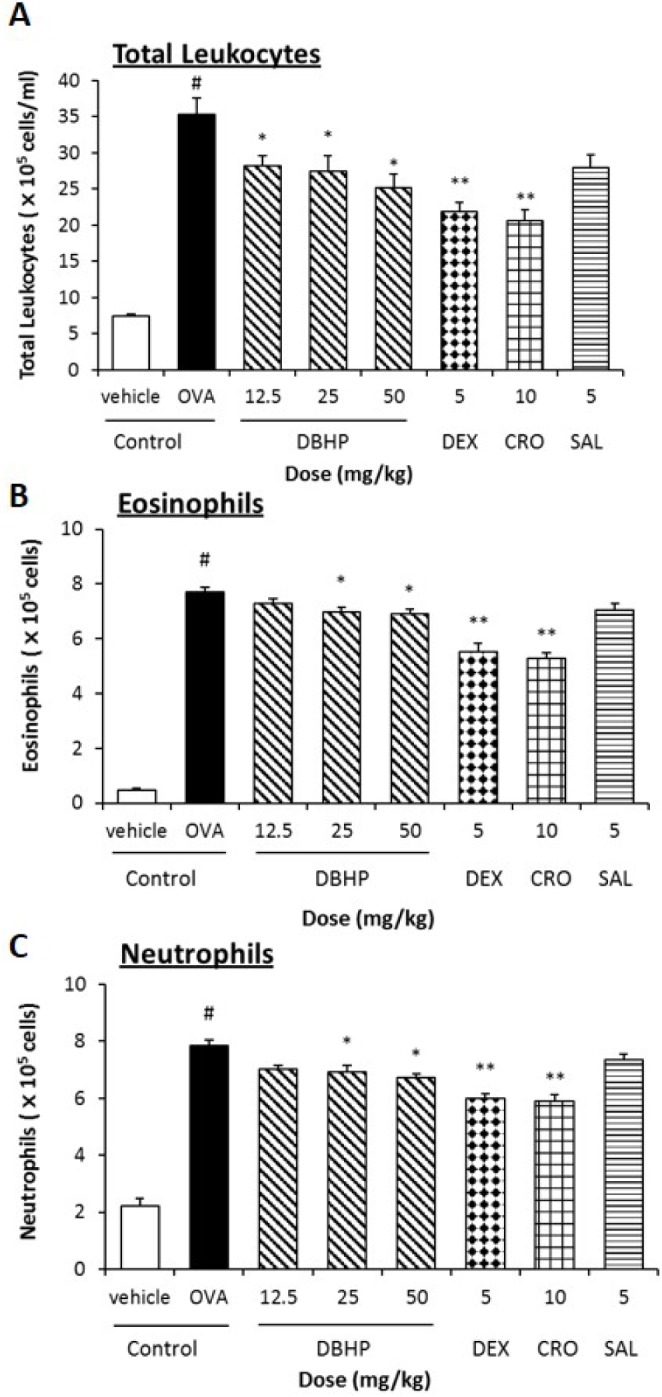 | Fig. 4Effect of DBHP on the recruitment of leukocytes in BALF.The number of total leukocytes (A), eosinophils (B), and neutrophils (C) were counted in BALF. Test drugs were orally administered 1 h prior to OVA challenge, additionally 12 h later. Each value represents the mean±SEM (n=6). Values are statistically significant at #p<0.05 compared with the vehicle control; *p<0.05 and **p<0.01 compared with OVA control. DBHP, 2,6-di-tert-butyl-4-hydroxymethylphenol; DEX, dexamethasone; CRO, cromoglycate; SAL, salbutamol.
|
Effect of DBHP on the release of biochemical inflammatory mediators in BALF
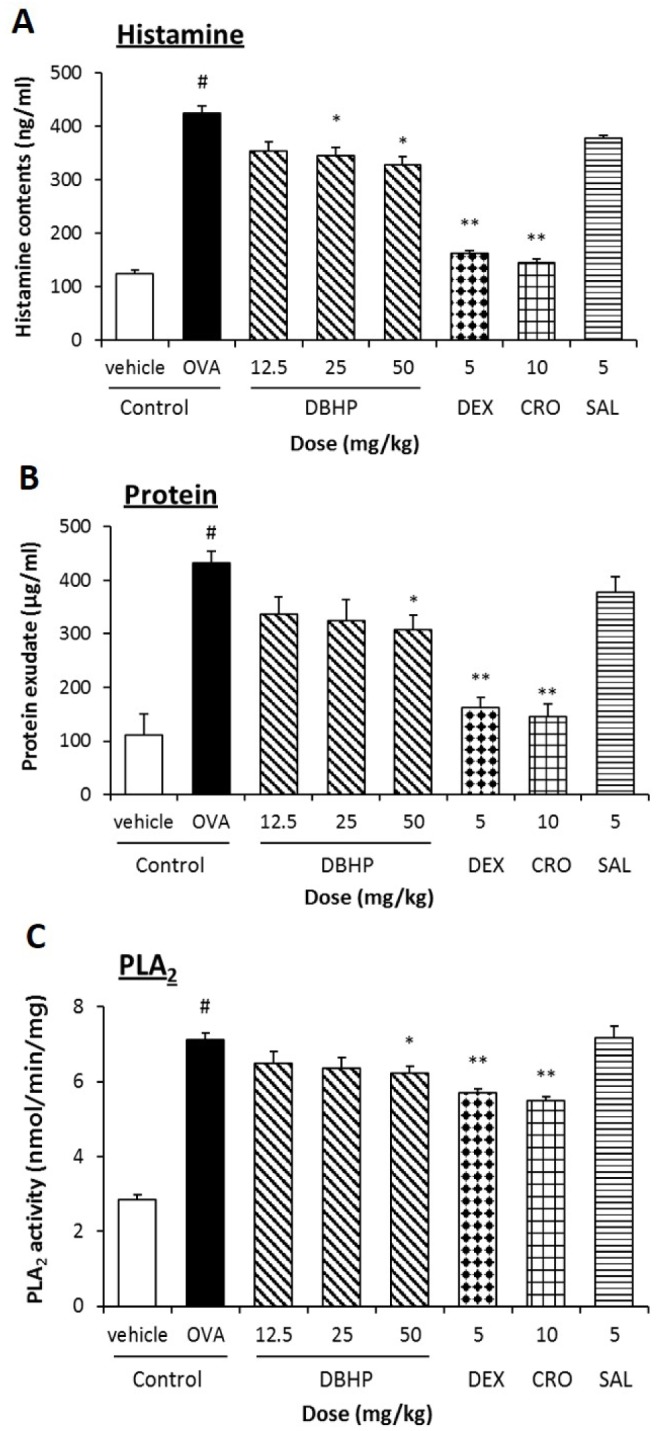 | Fig. 5Effect of DBHP on the release of biochemical mediators in BALF.Histamine content (A), protein exudates (B), and the specific activity of PLA2 (C) were measured fluorometrically and colorimetrically in BALF. Test drugs were orally administered 1 h prior to OVA challenge, additionally 12 h later. Each value represents the mean±SEM (n=6). Values are statistically significant at #p<0.05 compared with the vehicle control; *p<0.05 and **p<0.01 compared with OVA control. DBHP, 2,6-di-tert-butyl-4-hydroxymethylphenol; DEX, dexamethasone; CRO, cromoglycate; SAL, salbutamol.
|
Effect of DBHP on the histopathological changes in the asthmatic lung tissue
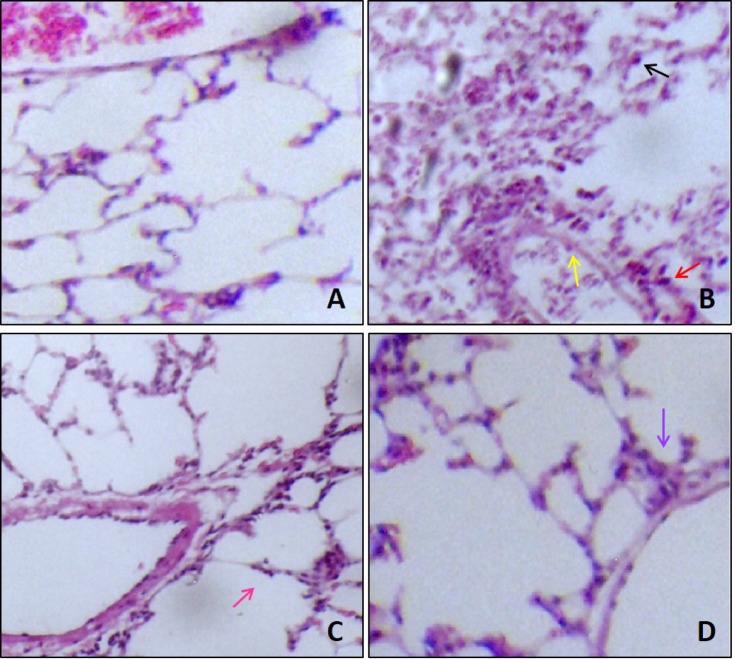 | Fig. 6Effect of DBHP on the histopathological survey in lung tissue.Representative images of the H&E-stained sections of lung tissue from; (A) vehicle control, showed a normal architecture and non-infiltration of inflammatory cells. (B) OVA control, exhibited the recruitment of leukocytes in alveolar sac (black arrow), peripheral vasculature (red arrow) and terminal bronchioles (yellow arrow). (C) asthmatic lung tissue treated with DBHP (50 mg/kg, p.o.) at 1 h before, and 12 h after OVA challenge, was reduced the recruitment of leukocytes into alveolar sacs (pink arrow). (D) asthmatic lung tissue treated with dexamethasone (5 mg/kg, p.o.) at 1 h before, and 12 h after OVA challenge, was also improved pathological changes with mild infiltration of leukocytes around bronchioles (purple arrow) (original magnification; ×100).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download