Abstract
Ethyl linoleate is an unsaturated fatty acid used in many cosmetics for its various attributes, such as antibacterial and anti-inflammatory properties and clinically proven to be an effective anti-acne agent. In this study, we investigated the effect of ethyl linoleate on the melanogenesis and the mechanism underlying its action on melanogenesis in B16F10 murine melanoma cells. Our results revealed that ethyl linoleate significantly inhibited melanin content and intracellular tyrosinase activity in α-MSH-induced B16F10 cells, but it did not directly inhibit activity of mushroom tyrosinase. Ethyl linoleate inhibited the expression of microphthalmia-associated transcription factor (MITF), tyrosinase, and tyrosinase related protein 1 (TRP1) in governing melanin pigment synthesis. We observed that ethyl linoleate inhibited phosphorylation of Akt and glycogen synthase kinase 3β (GSK3β) and reduced the level of β-catenin, suggesting that ethyl linoleate inhibits melanogenesis through Akt/GSK3β/β-catenin signal pathway. Therefore, we propose that ethyl linoleate may be useful as a safe whitening agent in cosmetic and a potential therapeutic agent for reducing skin hyperpigmentation in clinics.
The color of mammalian skin and hair is determined by melanin pigment [1]. Melanin pigment is synthesized in melanocytes and translocated to keratinocytes, protecting skin from hazardous environmental stimuli such as UV radiation [23]. Keratinocytes secrete α-melanocyte-stimulating hormone (α-MSH) to protect DNA damage caused by UV radiation [4]. α-MSH binds to melanocortin 1 receptor (MC1R) on melanocytes and stimulates the expression of microphthalmia-associated transcription factor (MITF) through cAMP-dependent pathway, which leads to melanogenesis [5]. However, excessive production of melanin results in hyperpigmentation, such as freckles, melasma, and dark spots [6]. Therefore, modulation of melanogenesis is a critical strategy for managing issues associated with abnormal skin pigmentation [7]. Melanin is synthesized via an enzymatic cascade controlled by tyrosinase, tyrosinase-related protein 1 (TRP1), and TRP2, leading to conversion of tyrosine to melanin pigments [8].
MITF is a central factor in melanogenesis that upregulates expression of tyrosinase, TRP1, and TRP2 [5]. MITF is regulated by transcription factors including MITF itself, cAMP-responsive element binding protein (CREB), paired box gene 3 (PAX3), sex determining region Y-box 10 (SOX10), lymphoid enhancer-binding factor 1/T-cell factor (LEF1/TCF), one cut domain 2 (ONECUT-2), and the mitogen-activated protein kinase (MAPK) pathway [59101112]. Recently, Wnt/β-catenin signal pathway was reported to play a significant role in melanin synthesis [1314]. Wnt ligand binds to cell surface Frizzled receptors, stabilizing β-catenin levels in the cell and ultimately resulting in interaction of β-catenin with LEF1/TCF and activation of the MITF promoter [15].
The various skin whitening agents, such as kojic acid, arbutin, and hydroquinone, have been developed to prevent or treat skin pigmentation. However, some of these agents have revealed harmful side effects, including skin irritation, carcinogenicity, genotoxicity, and oxidative damage to membrane lipids and proteins [1617181920]. For these reasons, some countries have placed restrictions on the use of these agents as cosmetic ingredients. Therefore, there has been an ongoing search for new treatment alternatives.
As stratum corneum, an outermost layer of the epidermis, is the rate-limiting barrier in absorption of an agent, how quickly chemical passes through this outer layer determines epidermis absorption [21]. Lipid-soluble chemicals penetrate the layer and into circulation faster and can be useful as a topical agent to prevent hyperpigmentary disorders such as melasma [222324]. Free fatty acids have been revealed to have regulatory effects on melanogenesis. For example, unsaturated fatty acid such as oleic acid (C18:1), linoleic acid (C18:2), and α-linolenic acid (C18:3) suppress melanin synthesis and tyrosinase activity, whereas saturated fatty acids such as palmitic acid (C16:0) and stearic acid (C18:0) induce melanin synthesis [252627].
Ethyl linoleate (linoleic acid ethyl ester), an unsaturated fatty acid resulting from formal condensation of the carboxyl group of linoleic acid with the hydroxyl group of ethanol, is used in many cosmetics for its antibacterial and anti-inflammatory properties [2829] and is reported to accelerate healing of wounds and clinically proven to be an effective anti-acne agent [30]. It was reported that the ethyl linoleate isolated from Oxalis triangularis inhibited melanogenesis by inhibiting tyrosinase promoter activity [31]. Though there are a few reports stating the anti-melanogenesis activity of ethyl linoleate, the underlying mechanism for tyrosinase modulation by ethyl linoleate is poorly understood. In this study, we examined the depigmentation effects of ethyl linoleate on α-MSH-induced melanogenesis and investigated the underlying molecular mechanisms of anti-melanogenesis. Understanding the molecular mechanisms of ethyl linoleate involved in depigmentation can lead to development of new skin brightening formulations that utilize Akt/GSK3β/β-catenin signal pathway.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), and 100× penicillin/streptomycin solution were obtained from Invitrogen Inc. (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were bought from Amresco Inc. (Solon, OH, USA). Ethyl linoleate, α-melanocyte-stimulating hormone (α-MSH), L-3,4-dihydroxyphenylalanine (L-DOPA), sodium hydroxide, mushroom tyrosinase, arbutin, kojic acid, and resveratrol were obtained from Sigma Chemical Co. (St. Louis, MO, USA). MITF, tyrosinase, and TRP1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and β-catenin antibody was purchased from BD (Franklin Lakes, NJ, USA). Akt, p-Akt, GSK3β, p-GSK3β, and β-actin antibodies were purchased from Cell signaling Technology (Beverly, MA, USA). Goat anti-mouse and -rabbit IgG secondary antibodies were obtained from Vector Laboratories (Burlingame, CA, USA).
The B16F10 murine melanoma cells were kindly obtained from Dr. Nam Ho Lee, Jeju National University, Korea. The human dermal fibroblast cells were kindly obtained from Dr. Moonjae Cho, Jeju National University, Korea. Both cell lines were cultured by using the DMEM containing 1% penicillin/streptomycin and 10% heat-inactivated FBS in a humidified atmosphere with 5% CO2 incubator at 37℃.
MTT assay was used to detect cell viability. The cells were seeded at a density of 4×103 cells/well for B16F10 and fibroblast cells on 96 well culture plates and cultured overnight. Cells were treated with various concentration of the ethyl linoleate for 48 h. Then, 20 µl of MTT reagent (5 mg/ml) was added to each well at 37℃ for 4 h. After removing the medium, the formazan crystals dissolved with DMSO (150 µl) and the absorbance was measured immediately at 570 nm using a microplate reader (Tecan, Grodig, Austria).
Melanin content was measured with a previously described method with slight modification [32]. After discarding the medium, the cells were harvested and centrifuged. The obtained pellet was dissolved by 1 N sodium hydroxide (NaOH) containing 10% DMSO at 80℃ for 30 min. The relative melanin production was determined by measuring the absorbance at 475 nm using the microplate reader.
In situ intracellular tyrosinase activity was conducted as previously reported method with minor modification [33]. Cells were fixed with 4% paraformaldehyde for 40 min, washed with PBS, and permeabilized with 0.1% Triton X-100 for 2 min. After washing the cells with PBS, cells were stained with 2 mM L-DOPA for 2 h at 37℃. Staining was imaged and analyzed using a camera attached to a microscope (Olympus, Essex, UK).
Intracellular tyrosinase activity was determined using a modified previously method [34]. Cells were collected and lysed in lysis buffer (100 mM Tris–HCl, pH 8, 250 mM NaCl, 0.5% Nonidet P-40, 1× protease inhibitor cocktail), sonicated several times, and then incubated on ice for 30 min. The lysates were centrifuged for 25 min at 14,000 rpm and 4℃, and the protein concentration of supernatants were determined by measuring with a BCA Protein Assay kit (Pierce, Rockford, IL, USA). The diluted lysates were mixed with 2 mM L-DOPA at 37℃, the absorbance was determined at 490 every 10 min for 1 h by a microplate reader.
Mushroom tyrosinase activity was used to investigate direct effects of ethyl linoleate on tyrosinase activity by using commercial tyrosinase isolated from mushrooms. The different diluted samples were mixed with 200 U/ml mushroom tyrosinase and 2 mM L-DOPA. After incubation at 37℃ for 10 min, the tyrosinase activity was determined by using the microplate reader at 490 nm absorbance.
Western blot was assessed according to the previously described [35]. Equal amounts of protein (10–40 µg) from the cultures were separated by 10–12% SDS-PAGE and transferred onto PVDF membrane (Millipore, Billerica, MA, USA). Membranes were probed with anti-MITF (1:1,000), tyrosinase (1: 7,000), TRP1 (1: 7,000), Akt (1: 2,000), p-Akt (1: 1,000), GSK3β (1: 2,000), p-GSK3β (1: 1,000), β-catenin (1: 2,000), and β-actin (1: 10,000). The membranes were incubated with the secondary antibody at a 1:5,000 dilutions, and protein bands were detected by using the BS ECL plus kit (Biosesang, Gyeonggi-do, Korea). Relative the band intensity was analyzed using ImageJ analysis software (National Institutes of Health, Bethesda, MD, USA).
Cytotoxicity of a drug is critical if the drug is used either as a cosmetic or as a medicine agent [3637]. To examine the cell safety of ethyl linoleate, we treated B16F10 murine melanoma and human dermal fibroblast cells with various concentration of ethyl linoleate. As shown in Fig. 1A and B, ethyl linoleate revealed no significant cytotoxic effect on both cell types at a concentration of 400 µM. Therefore, we used 400 µM ethyl linoleate for further experiments. To evaluate effects of ethyl linoleate on melanogenesis in B16F10 cells, cells were stimulated by propigmenting agents α-MSH in the presence or absence of ethyl linoleate for 48 h. The excessive melanin production with α-MSH treatment was significantly inhibited by ethyl linoleate co-treatment (Fig. 1C). When cells were cultured in medium containing ethyl linoleate, the stimulation of melanin production by α-MSH was reduced to 30.40% by 400 µM ethyl linoleate. According to these results, ethyl linoleate is a noncytotoxic anti-melanogenesis chemical. Therefore, it was studied to evaluate its molecular mechanisms underlying anti-melanogenesis effect by using B16F10 cells.
As tyrosinase is the rate-limiting enzyme critical for melanin biosynthesis, inhibitors of tyrosinase activity have been sought as therapeutic means to treat hyperpigmentary disorders and cosmetic agent [38]. To elucidate ethyl linoleate-mediated inhibition of tyrosinase activity, cells were incubated with L-DOPA, that detected in situ intracellular tyrosinase activity. Treatment with ethyl linoleate for 48 h reduced in situ intracellular tyrosinase activity without affecting cell viability compared to that in the α-MSH control (Fig. 2A). In addition, Fig. 2B revealed that ethyl linoleate treatment induced inhibition of intracellular tyrosinase activity compared to that in the α-MSH control. Whereas, under cell-free conditions, ethyl linoleate revealed no notable inhibitory effect on the activity of mushroom tyrosinase activity (Fig. 2C), suggesting that the effect on B16F10 cells was not mediated by direct interaction of ethyl linoleate with the tyrosinase enzyme. Data indicated that ethyl linoleate probably inhibited the function of tyrosinase by decreasing tyrosinase expression.
As ethyl linoleate treatment induced inhibition of intracellular tyrosinase activity, not mushroom tyrosinase activity, we determined levels of melanogenic enzyme proteins, such as tyrosinase and tyrosinase-related protein 1 (TRP1). Fig. 3A shows that protein expressions of tyrosinase and TRP1 were repressed with increasing concentration of ethyl linoleate. Quantification of the detected signals revealed that protein levels of tyrosinase and TRP1 following treatment with 400 µM ethyl linoleate were reduced to 50.02% and 63.17% of the α-MSH control, respectively (Figs. 3B and C). While resveratrol, a tyrosinase inhibitor, only reduced expression of tyrosinase, not TRP1, suggesting that ethyl linoleate treatment reduced synthesis of melanin more than resveratrol treatment due to the reduction in the expression of both tyrosinase and TRP1.
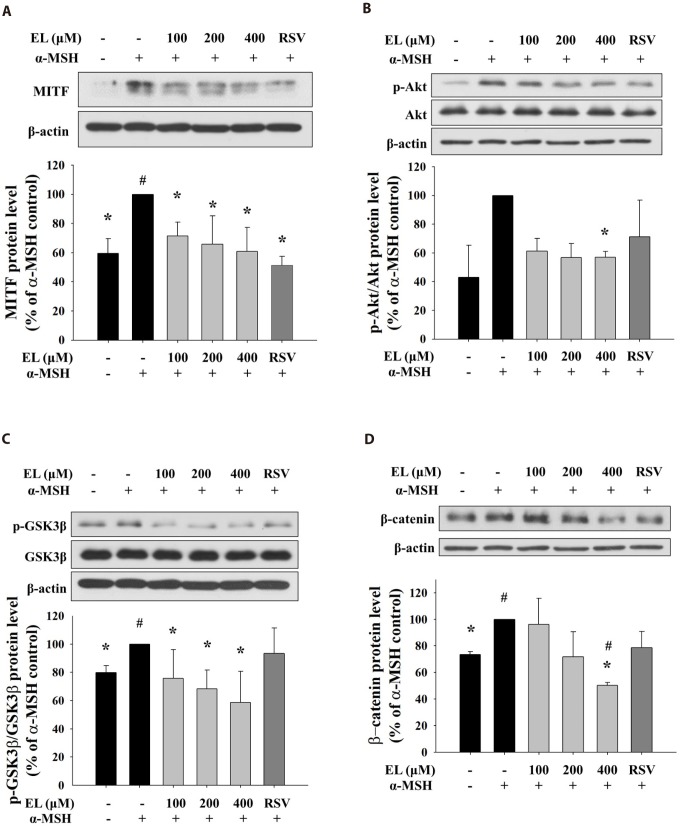
To evaluate the mechanism of the inhibitory action of ethyl linoleate on melanogenesis, we treated cells with various concentration of ethyl linoleate with α-MSH and examined the involvement of Akt/GSK3β/β-catenin signaling pathway. The microphthalmia-associated transcription factor (MITF) is widely regarded as the key transcriptional regulator of melanogenic enzyme proteins. As shown in Fig. 4A, the expression of MITF was reduced in a dose-dependent manner by 71.49%, 65.73%, and 60.80% in the presence of 100, 200, and 400 µM ethyl linoleate, respectively, compared to α-MSH alone treatment. Ethyl linoleate decreased the ratio of phosphorylated-form/total-form of Akt and GSK3β by 56.96% and 58.54% in the presence of 400 µM ethyl linoleate, respectively, compared to α-MSH alone (Fig. 4B, C). Furthermore, ethyl linoleate inhibited level of expression of β-catenin in a concentration-dependent manner by 96.23%, 71.67%, and 50.31% in the presence of 100, 200, and 400 µM ethyl linoleate, respectively, compared to α-MSH alone treatment (Fig. 4D). According to these results, hyperpigmentation inhibition by ethyl linoleate is associated with inhibition of phosphorylation of Akt and GSK3β that led to suppression of MITF expression through degradation of β-catenin, reduced tyrosinase and TRP1 expression, and inhibited melanin production.
To further confirm involvement of Akt/GSK3β/β-catenin signaling pathway in ethyl linoleate-induced inhibition of melanogenesis, we conducted a time course experiment to establish the kinetic of melanogenesis-related proteins expression following 400 µM ethyl linoleate treatment in α-MSH stimulation. As shown in supplementary Fig. 1, α-MSH treatment markedly increased levels of tyrosinase and TRP1 in a time-dependent manner. MITF expression was increased after α-MSH treatment, which peaked at 4 h, followed by a continuous decline to 24 h, and a recovery to basal levels after 48 h of treatment. The ethyl linoleate co-treatment with α-MSH began to decrease the protein level of MITF at 4 h, which continued decline to 12 h. In addition, we observed that the phosphorylated Akt by ethyl linoleate rapidly declined more than those by α-MSH only. The ethyl linoleate cotreatment with α-MSH caused decrease in the phosphorylation of GSK3β levels; however, there was no significant reduction in the phosphorylation of GSK3β following the α-MSH alone treatment. It was also apparent that ethyl linoleate co-treatment with α-MSH resulted in the loss of β-catenin expression from 4 h to 48 h.
Many investigations have focused on the specific mechanisms involved in melanogenesis to develop new therapeutic agents for skin pigmentation abnormalities. Pigmentation may be regulated by different steps: expression of melanogenic enzyme proteins, regulation of melanogenic enzyme activity during or before melanin synthesis, melanosome transfer to recipient keratinocytes, and melanosome degradation and turnover. Among these steps, the expression of melanogenic enzyme proteins is the most important step for regulation of pigmentation [39]. Tyrosinase, tyrosinase-related protein 1 (TRP1), and TRP2 are key enzymes for melanin biosynthesis, and these enzymes are influenced by microphthalmia-associated transcription factor (MITF) [5].
Previous studies have been reported that unsaturated fatty acids, such as oleic acid, linoleic acid, and α-linolenic acid, suppress melanin biosynthesis through the inhibition of tyrosinase activities. It has revealed that linoleic acid enhances the ubiquitination of mature tyrosinase and the ubiquitinated tyrosinase could be integrated in the ER-associated degradation after rapid processing of tyrosinase from the ER to the Golgi [2627]. However, the underlying mechanism for tyrosinase modulation by ethyl linoleate is poorly understood thus far. In the present study, we observed that ethyl linoleate decreased the expression of tyrosinase and TRP1 through the reduction of MITF expression as shown in Fig. 4. Furthermore, we found that hyperpigmentation inhibition by ethyl linoleate is associated with inhibition of phosphorylation of Akt and GSK3β that led to suppression of MITF expression through degradation of β-catenin in a concentration dependent manner.
It is known that MITF is regulated by the balance between a variety of signal transduction pathway, including the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA), extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK), and Akt pathway [1240414243]. While we could not detect changes in phosphorylation of CREB, ERK, JNK, and p38 (data not shown), phosphorylation of Akt was decreased compared to α-MSH control (Fig. 4B). It has been reported that activated Akt can phosphorylate glycogen synthase kinase 3β (GSK3β) [44]. When GSK3β is phosphorylated, GSK3β-dependent phosphorylation of β-catenin is blocked and β-catenin is translocated into the nucleus, that recruits the complex of β-catenin and LEF1/TCF to binding sites of the MITF promoter [1445]. Our data showed that ethyl linoleate treatment decreased the expression of p-Akt, p-GSK3β, and β-catenin (Figs. 4B-D). Therefore, the antimelanogenesis effect by ethyl linoleate contributed to suppression of MITF expression through Akt/GSK3β/β-catenin signaling pathway.
In conclusion, ethyl linoleate decreased melanin production and tyrosinase activity through the reduction of tyrosinase and TRP1 expression. Our data revealed that ethyl linoleate treatment decreased expression of MITF through Akt/GSK3β/β-catenin pathway. This study provides a molecular basis for understanding inhibitory effects of ethyl linoleate on melanogenesis. Ethyl linoleate may be used as a non-cytotoxic and skin-whitening agent as a cosmetic and medicine.
ACKNOWLEDGEMENTS
This research was supported by the 2017 scientific promotion program funded by Jeju National University.
Notes
References
1. Clarys P, Alewaeters K, Lambrecht R, Barel AO. Skin color measurements: comparison between three instruments: the Chromameter®, the DermaSpectrometer® and the Mexameter®. Skin Res Technol. 2000; 6:230–238. PMID: 11428962.

2. Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002; 12:405–416. PMID: 12394181.
3. Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, Koulisis N, Farrel C, Amos CI, Wei Q, Lee JE, Zhang J, Kupper TS, Qureshi AA, Cui R, Han J, Fisher DE, Arany Z. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell. 2013; 49:145–157. PMID: 23201126.

4. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007; 128:853–864. PMID: 17350573.

5. Hsiao JJ, Fisher DE. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch Biochem Biophys. 2014; 563:28–34. PMID: 25111671.

6. Speeckaert R, Van Gele M, Speeckaert MM, Lambert J, van Geel N. The biology of hyperpigmentation syndromes. Pigment Cell Melanoma Res. 2014; 27:512–524. PMID: 24612852.

7. Chung KW, Jeong HO, Jang EJ, Choi YJ, Kim DH, Kim SR, Lee KJ, Lee HJ, Chun P, Byun Y, Moon HR, Chung HY. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim Biophys Acta. 2013; 1830:4752–4761. PMID: 23769841.

8. Hearing VJ. Determination of melanin synthetic pathways. J Invest Dermatol. 2011; 131:E8–E11. PMID: 22094404.

9. Jacquemin P, Lannoy VJ, O'Sullivan J, Read A, Lemaigre FP, Rousseau GG. The transcription factor onecut-2 controls the microphthalmia-associated transcription factor gene. Biochem Biophys Res Commun. 2001; 285:1200–1205. PMID: 11478782.

10. Saito H, Yasumoto K, Takeda K, Takahashi K, Fukuzaki A, Orikasa S, Shibahara S. Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with lymphoid-enhancing factor 1. J Biol Chem. 2002; 277:28787–28794. PMID: 12048204.

11. Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Le Caignec C, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000; 9:1907–1917. PMID: 10942418.

12. Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, Fisher DE. α-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem. 1998; 273:33042–33047. PMID: 9830058.

13. Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci. 2015; 79:74–83. PMID: 25869056.

14. Hwang I, Park JH, Park HS, Choi KA, Seol KC, Oh SI, Kang S, Hong S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013; 72:274–283. PMID: 24016750.

15. Bellei B, Pitisci A, Catricala C, Larue L, Picardo M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation. Pigment Cell Melanoma Res. 2011; 24:309–325. PMID: 21040502.

16. Nakagawa M, Kawai K, Kawai K. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995; 32:9–13. PMID: 7720390.

17. Takizawa T, Imai T, Onose J, Ueda M, Tamura T, Mitsumori K, Izumi K, Hirose M. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol Sci. 2004; 81:43–49. PMID: 15201437.
18. Cheng SL, Liu RH, Sheu JN, Chen ST, Sinchaikul S, Tsay GJ. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J Biomed Sci. 2007; 14:87–105. PMID: 17103032.

19. Bolognia JL, Sodi SA, Osber MP, Pawelek JM. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br J Dermatol. 1995; 133:349–357. PMID: 8546987.

20. Makino ET, Mehta RC, Banga A, Jain P, Sigler ML, Sonti S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol. 2013; 12:s16–s20. PMID: 23545928.
21. Baynes RE, Hodgson E. Absorption and distribution of toxicants. In : Hodgson E, editor. A textbook of modern toxicology. 3rd ed. New Jersey: John Wiley & Sons;2004. p. 75–110.
22. Lee MH, Kim HJ, Ha DJ, Paik JH, Kim HY. Therapeutic effect of topical application of linoleic acid and lincomycin in combination with betamethasone valerate in melasma patients. J Korean Med Sci. 2002; 17:518–523. PMID: 12172049.

23. Thirion L, Piérard-Franchimont C, Piérard GE. Whitening effect of a dermocosmetic formulation: a randomized double-blind controlled study on melasma. Int J Cosmet Sci. 2006; 28:263–267. PMID: 18489266.

24. Morganti P, Ruocco E, Wolf R, Ruocco V. Percutaneous absorption and delivery systems. Clin Dermatol. 2001; 19:489–501. PMID: 11535394.
25. Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res. 1998; 290:375–381. PMID: 9749992.
26. Ando H, Watabe H, Valencia JC, Yasumoto K, Furumura M, Funasaka Y, Oka M, Ichihashi M, Hearing VJ. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004; 279:15427–15433. PMID: 14739285.
27. Ando H, Wen ZM, Kim HY, Valencia JC, Costin GE, Watabe H, Yasumoto K, Niki Y, Kondoh H, Ichihashi M, Hearing VJ. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem J. 2006; 394:43–50. PMID: 16232122.

28. Park SY, Seetharaman R, Ko MJ, Kim DY, Kim TH, Yoon MK, Kwak JH, Lee SJ, Bae YS, Choi YW. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int Immunopharmacol. 2014; 19:253–261. PMID: 24508058.

29. Jelenko C, Wheeler ML, Anderson AP, Callaway BD, McKinley JC. Studies in burns: XIV, Heling in burn wounds treated with Ethyl Linoleate alone or in combination with selected topical antibacterial agents. Ann Surg. 1975; 182:562–566. PMID: 1190861.
30. Charakida A, Charakida M, Chu AC. Double-blind, randomized, placebo-controlled study of a lotion containing triethyl citrate and ethyl linoleate in the treatment of acne vulgaris. Br J Dermatol. 2007; 157:569–574. PMID: 17635508.

31. Huh S, Kim YS, Jung E, Lim J, Jung KS, Kim MO, Lee J, Park D. Melanogenesis inhibitory effect of fatty acid alkyl esters isolated from Oxalis triangularis. Biol Pharm Bull. 2010; 33:1242–1245. PMID: 20606321.

32. Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α,25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985; 45:1474–1478. PMID: 2983883.
33. Newton RA, Cook AL, Roberts DW, Leonard JH, Sturm RA. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J Invest Dermatol. 2007; 127:2216–2227. PMID: 17460731.

34. Takahashi H, Parsons PG. Rapid and reversible inhibition of tyrosinase activity by glucosidase inhibitors in human melanoma cells. J Invest Dermatol. 1992; 98:481–487. PMID: 1532183.

35. Song YW, Cho SK. Phytol induces apoptosis and ROS-mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem Anal Biochem. 2015; 4:4.

36. Lehman AJ, Patterson WI, Davidow B, Hagan EC, Woodard G, Laug EP, Frawley JP, Fitzhugh OG, Bourke AR, Draize JH, Nelson AA, Vos BJ. Procedures for the appraisal of the toxicity of chemicals in foods, drugs and cosmetics. Food Drug Cosmet Law J. 1955; 10:679–748.
37. Tomankova K, Kejlova K, Binder S, Daskova A, Zapletalova J, Bendova H, Kolarova H, Jirova D. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol In Vitro. 2011; 25:1242–1250. PMID: 21570462.

38. Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007; 127:751–761. PMID: 17218941.

39. Park KC, Huh SY, Choi HR, Kim DS. Biology of melanogenesis and the search for hypopigmenting agents. Dermatologica Sinica. 2010; 28:53–58.

40. Oka M, Nagai H, Ando H, Fukunaga M, Matsumura M, Araki K, Ogawa W, Miki T, Sakaue M, Tsukamoto K, Konishi H, Kikkawa U, Ichihashi M. Regulation of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway in human G361 melanoma cells. J Invest Dermatol. 2000; 115:699–703. PMID: 10998146.

41. Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000; 14:301–312. PMID: 10673502.

42. Mansky KC, Sankar U, Han J, Ostrowski MC. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-κB ligand signaling. J Biol Chem. 2002; 277:11077–11083. PMID: 11792706.

43. Bu J, Ma PC, Chen ZQ, Zhou WQ, Fu YJ, Li LJ, Li CR. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am J Chin Med. 2008; 36:245–263. PMID: 18457359.

44. Khaled M, Larribere L, Bille K, Ortonne JP, Ballotti R, Bertolotto C. Microphthalmia associated transcription factor is a target of the phosphatidylinositol-3-kinase pathway. J Invest Dermatol. 2003; 121:831–836. PMID: 14632202.

45. Hart MJ, de los, Albert IN, Rubinfeld B, Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998; 8:573–581. PMID: 9601641.

SUPPLEMENTARY MATERIALS
Supplementary data including one figure can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp022-01-06-s001.pdf.
Supplementary Fig. 1
Effects of ethyl linoleate on expression of melanogenesis-related proteins in B16F10 cells.
Fig. 1
Effects of cell cytotoxicity and melanin production of ethyl linoleate.
The cells were treated for 48 h with indicated concentration of ethyl linoleate. Cell viabilities were determined by MTT assay on B16F10 murine melanoma (A) and human dermal fibroblast cells (B). (C) The B16F10 cells were exposed to α-melanocyte-stimulating hormone (α-MSH, 500 nM) in the presence of ethyl linoleate (EL) for 48 h. Melanin content in B16F10 cells was visualized and determined at the indicated concentrations of EL or positive whitening agents (AR, arbutin 2 mM; KA, kojic acid 400 µM; RSV, resveratrol 20 µM). The data are expressed as the means±SD. *p<0.01, compared with the α-MSH control; #p<0.01, compared with the vehicle control.

Fig. 2
Effects of ethyl linoleate on tyrosinase activity in B16F10 cells.
The B16F10 cells were exposed to α-melanocyte-stimulating hormone (α-MSH, 500 nM) in the presence of ethyl linoleate (EL) for 48 h. (A) In situ intracellular tyrosinase activity determined by L-DOPA staining. Resveratrol (RSV, 20 µM) was used as a positive control. Images were captured under identical conditions using bright field microscopy. Bar=20 µm. (B) Intracellular tyrosinase activity was determined using lysates obtained from B16F10 cells treated with EL or RSV. (C) The direct effect of ethyl linoleate on tyrosinase activity was measured with mushroom tyrosinase. Kojic acid (KA, 400 µM) was used as a positive control. The data are expressed as the means±SD. *p<0.01, compared with the α-MSH control; #p<0.01, compared with the vehicle control.
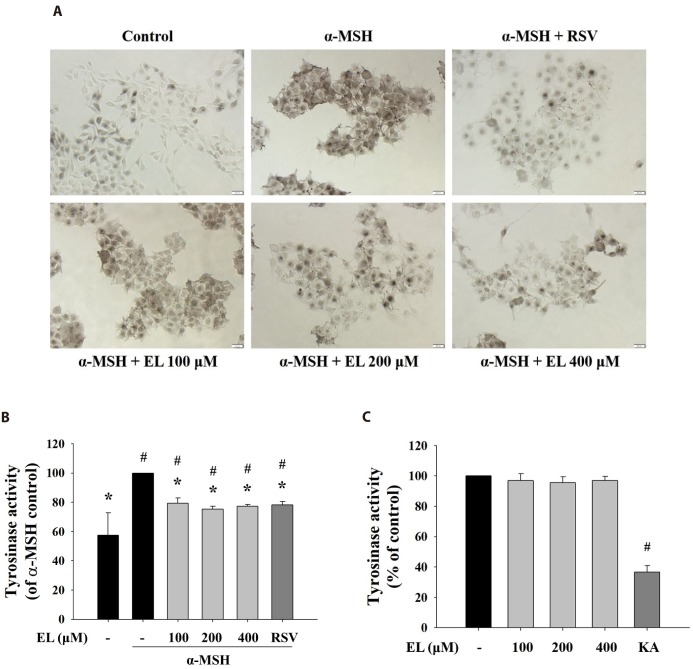
Fig. 3
Effects of ethyl linoleate on expression of melanogenic enzyme proteins in B16F10 cells.
The B16F10 cells were exposed to α-melanocyte-stimulating hormone (α-MSH, 500 nM) in the presence of ethyl linoleate (EL) for 48 h. (A) The levels of tyrosinase, TRP1, and β-actin from ethyl linoleate treated B16F10 cells were detected using western blot. Band intensity of tyrosinase (B) and TRP1 (C) compared to the α-MSH control was determined by ImageJ software. Resveratrol (RSV, 20 µM) was used as a positive control. The data are expressed as the means±SD. *p<0.05, compared with the α-MSH control; #p<0.05, compared with the vehicle control.
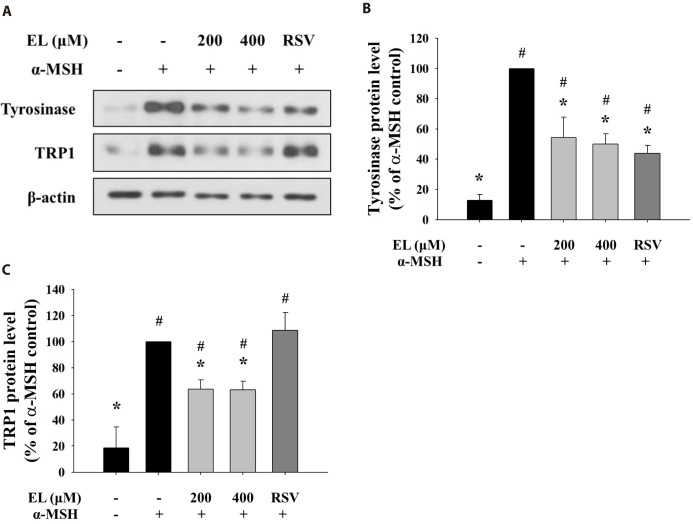
Fig. 4
Effects of ethyl linoleate on expression of MITF through Akt/GSK3β/β-catenin signal in B16F10 cells.
The B16F10 cells were exposed to α-melanocyte-stimulating hormone (α-MSH, 500 nM) in the presence of ethyl linoleate (EL) or resveratrol (RSV, 20 µM) for 4 h. The expression levels of protein including Akt, p-Akt, GSK3β, p-GSK3β, β-catenin, and β-actin were detected by using western blot. Band intensity compared to the α-MSH control was determined by ImageJ software. (A) MITF, (B) Akt and p-Akt, (C) GSK3β and p-GSK3β, and (D) β-catenin. The data are expressed as the means±SD. *p<0.05, compared with the α-MSH control; #p<0.05, compared with the vehicle control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download