Abstract
Adenosine is a naturally occurring breakdown product of adenosine triphosphate and plays an important role in different physiological and pathological conditions. Adenosine also serves as an important trigger in ischemic and remote preconditioning and its release may impart cardioprotection. Exogenous administration of adenosine in the form of adenosine preconditioning may also protect heart from ischemia-reperfusion injury. Endogenous release of adenosine during ischemic/remote preconditioning or exogenous adenosine during pharmacological preconditioning activates adenosine receptors to activate plethora of mechanisms, which either independently or in association with one another may confer cardioprotection during ischemia-reperfusion injury. These mechanisms include activation of KATP channels, an increase in the levels of antioxidant enzymes, functional interaction with opioid receptors; increase in nitric oxide production; decrease in inflammation; activation of transient receptor potential vanilloid (TRPV) channels; activation of kinases such as protein kinase B (Akt), protein kinase C, tyrosine kinase, mitogen activated protein (MAP) kinases such as ERK 1/2, p38 MAP kinases and MAP kinase kinase (MEK 1) MMP. The present review discusses the role and mechanisms involved in adenosine preconditioning-induced cardioprotection.
Go to : 
Adenosine is a naturally occurring breakdown product of adenosine triphosphate (ATP), which is hydrolyzed and dephosphorylated to produce adenosine. It may be produced inside the cells (intracellular) by the action of enzyme 5′-nucleotidase on 5′-AMP. On the other hand, adenosine in the extracellular space is formed by the action of enzyme ecto-5′ nucleotidase [1]. Apart from a simple degradation product, adenosine produces multiple physiological effects such as regulation of blood flow, heart rate and heart contractility through activation of different adenosine receptors [23]. Four types of adenosine receptors, A1, A2A, A2B and A3 have been identified and all are G-protein coupled receptors. The A1 and A3 are Gi-coupled receptors that inhibit adenylyl cyclase activity and cAMP production; whereas, A2A and A2B are Gs-coupled receptors that activates adenylyl cyclase and cAMP production. Furthermore, A2B receptors also couple to Gq proteins to activate phospholipase C [45]. Adenosine receptors are widely expressed in the body. The A1 receptors are highly expressed throughout the CNS, heart muscles and in inflammatory cells [6]. The A2 receptors are located in pre- and postsynaptic nerve terminals, mast cells, heart (smooth muscle and endothelial cells) and in circulating leukocytes. A3 receptors are found in the heart (endothelial cells of the aorta, smooth muscle cells), kidney, testis, mast cells, eosinophils, neutrophils and in brain cortex [17].
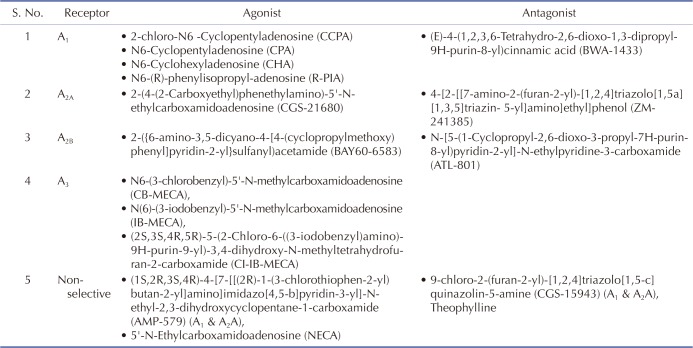
Ischemic preconditioning is a powerful tool in which brief episodes of ischemia and reperfusion protect the heart from subsequent prolonged ischemia [8]. Various authors have demonstrated that adenosine may be involved in ischemic preconditioning [910]. During ischemic preconditioning, the increased level of adenosine in coronary blood samples (venous and arterial) and in perfusate samples from the heart has been reported [111213]. Moreover, the key role of adenosine has also been elucidated in remote ischemic preconditioning, which is a phenomenon in which brief episodes of ischemia to organ other than heart (remote organ) confers protection to heart [1415]. To elucidate the mechanisms involved in adenosine mediated cardioprotection in ischemic as well as remote preconditioning, scientists administered adenosine exogenously in the form of adenosine preconditioning (pharmacological preconditioning) [16]. Scientists have employed selective agonist and antagonists of different adenosine receptors to explore the mechanisms involved in adenosine preconditioning (Table 1). Studies have shown that adenosine induces cardioprotective effects by various mechanisms that include activation of KATP channels [171819], increase in antioxidant enzymes (glutathione peroxidase, catalase) [2021], interaction with opioid receptors [22], various kinases (PKC, MAP Kinase, MEK 1, ERK 1/2 and tyrosine kinase) [232425262728] and increase in nitric oxide level [19]. The present review describes these mechanisms involved in adenosine-induced preconditioning.
Go to : 
It is very well documented that there is a release of adenosine during ischemic preconditioning, which plays a key role in inducing cardioprotection [1129]. It is shown that first 5 min after its endogenous release (or its exogenous administration) are very important and this short period serves as an important time window for inducing cardioprotection [30]. It is also reported that intracellular formation of adenosine from breakdown of ATP is significant in triggering cardioprotective actions of ischemic preconditioning. On the other hand, it is stated that extracellular formation of adenosine using ecto-5′-nucleotidase plays a negligible role in preventing ischemic injury to heart [31]. It is also shown that there may be activation of the vagal system during ischemic preconditioning [32], which may be important in increasing the intracellular levels of adenosine [33].
There have a number of studies showing that remote preconditioning-induced cardioprotective effects may also be mediated through release of adenosine. Scientists have employed different strategies to elucidate the role of adenosine in remote preconditioning such as employment of specific adenosine receptor antagonists before remote preconditioning stimulus [3435] or employment of transgenic animals with targeted ablation of A1 receptors [36]. The attenuated effects of remote preconditioning in such type of transgenic animals or in the presence of adenosine blockers provide the evidence that adenosine plays a key role in remote preconditioning [15]. Leung et al. demonstrated that coronary effluent, collected from isolated perfused rabbit hearts following ischemic preconditioning stimulus, is capable of producing cardioprotection in another animal in the form of remote preconditioning. However, adenosine receptor blocker abolished remote preconditioning effects of coronary effluent suggesting adenosine may be the cardioprotective humoral factor, which may be transferred via coronary effluent [37]. Based on these studies, scientists have proposed that adenosine is the key humoral factor, which is released in the blood during remote preconditioning episodes.
Go to : 
Exogenous administration of adenosine (or adenosine receptor agonists) in the form of pharmacological preconditioning has also been shown to mimic the cardioprotective effects of ischemic and remote preconditioning [202138]. A study from our laboratory demonstrated that administration of adenosine in rats attenuates ischemia-reperfusion-induced injury in isolated rat heart on Langendorff system [16]. Intravenous administration of adenosine is shown to protect the heart injury following intestinal ischemia reperfusion injury in rats [39]. A potential advantage of pharmacological preconditioning over ischemic preconditioning is that exogenous administration of pharmacological agents (say adenosine) may elicit preconditioning like effects in situations, where the efficacy of ischemic preconditioning is reduced. It is well documented that the cardioprotective effects of ischemic preconditioning are significantly abolished in angina pectoris and in diabetic patients. However, a clinical study of Shehata demonstrated the efficacy of adenosine preconditioning in patients suffering from diabetes mellitus and stable angina pectoris. Intracoronary administration of adenosine (100 µg/stented vessel) was shown to attenuate cardiac injury in diabetic patients (n=100) with chronic stable angina undergoing elective percutaneous coronary intervention [40].
Go to : 
There have been a number of studies documenting that adenosine produces its cardioprotective effects through KATP channels. Baxter and Yellon reported that A1 receptor-induced cardioprotection is mediated through opening of KATP channels. Pretreatment of rabbits with CCPA (A1 receptor agonist) (0.1 mg/kg i.v.) 24 h before 30 min of coronary artery occlusion and 120 min of reperfusion reduced myocardial infarct size. Treatment with glibenclamide (0.3 mg/kg) or 5-HD (5-hydroxydecanoate) (5 mg/kg) (KATP channel blockers) completely abolished the cardioprotective effect of CCPA suggesting that KATP channel opening mediates A1 receptor-induced cardioprotection [17]. Tracey et al elucidated the role of KATP channel opening in A3 receptor mediated cardioprotection. Perfusion of isolated rabbit heart with selective A3 receptor agonist CB-MECA (N6-(3-chlorobenzyl)-5-N-methylcarboxamidoadenosine) before 30 min of regional ischemia and 120 min of reperfusion, produced the infarct limiting effects. Pretreatment with dose dependent A1 receptor antagonist (BWA 1433) (50 nM) did not alter the infarct limiting effect of CB-MECA, confirming that CB-MECA elicited the infarct limiting effect via A3 receptor activation. Furthermore, pretreatment with glibenclamide and 5-HD abolished the infarct limiting effects of A3 receptor agonist (CB-MECA). This suggests that A3 receptor activation produces infarct limiting effect via opening of KATP channels [18].
There have been other reports elucidating the key role of KATP channel opening in adenosine receptor mediated cardioprotection. Takano and coworkers demonstrated that pretreatment with CCPA (A1 receptor agonist) or IB-MECA (A3 receptor agonist), 24 h before 30 min of coronary artery occlusion and 72 h of reperfusion in conscious rabbits reduced the infarct size. There was no effect of CGS-21680 (A2A receptor antagonist) suggesting that A1 and A3 receptors (not A2A receptors) are involved in late phase of ischemic preconditioning. Treatment with 5-HD immediately before 30 min of coronary artery occlusion, completely abrogated the infarct limiting effect of CCPA and IB-MECA. This revealed that A1 and A3 receptor activation produced cardioprotective effects via KATP channel opening [19]. A study of Gopalakrishnan also reported that treatment of guinea pig bladder cells with adenosine opens KATP channels in same way as treatment with P1075 (KATP channel opener) [41], further supporting that adenosine produces effects via opening of KATP channels. Yang et al demonstrated that perfusion of mouse hearts with adenosine (100 µM), as pharmacological preconditioning, significantly attenuated ischemic injury by preserving the surface expression of KATP channels subunits. Preconditioning with adenosine significantly prevented ischemia-induced decrease in sarcolemmal KATP channel expression and internalization of KATP channels to endosomal compartments [42].
Adenosine may also produce its cardioprotective effects by increasing the levels of antioxidant enzymes. Dana et al reported an increase in Mn-SOD (Manganese superoxide dismustase) activity in response to A1 receptor activation. Pretreatment of rats with A1 receptor agonist (CCPA) (75 mg/kg i.v. bolus), 24 h before 35 min of regional ischemia and 2 h of reperfusion, decreased the infarct size. The infarct limiting effect of CCPA was abolished by AS-ODN (antisense oligonucleotides to Mn-SOD) revealing that A1 receptor activation enhances the Mn-SOD activity to produce cardioprotective effects [20]. Hochhauser et al. demonstrated that perfusion with CCPA (A1 receptor agonist) and CI-IB-MECA (A3 receptor agonist) before ischemia-reperfusion increased that levels of antioxidant enzymes including SOD, catalase and glutathione peroxidase. It suggests that A1 and A3 receptor activation may increase the level of antioxidant enzymes to produce cardioprotection [21]. Other studies have also shown that adenosine receptor activation increases the antioxidant levels. Husain and Somani demonstrated that treatment of rats with A1 receptor agonist, R-phenyl isopropyl adenosine (R-PIA), increases the levels of SOD, catalase, glutathione peroxidase and reduced glutathione. Furthermore, pretreatment with theophylline (non-selective adenosine receptor blocker) blocked R-PIA-induced increase in antioxidant enzyme levels, suggesting that adenosine receptor activation increases the antioxidant enzyme levels [43]. In another study, it is demonstrated that treatment of cultured endothelial cells and cardiomyocytes with R-PIA (A1 receptor agonist) increases the levels of SOD, catalase and glutathione peroxidase. Pretreatment with theophylline reversed R-PIA-induced increase in antioxidant enzyme levels, suggesting that A1 receptor activation may increases the levels of antioxidant enzymes [44].
There have studies showing that adenosine may increase the levels of NO to produce cardioprotective effects. Furthermore, there have been studies showing that increase in nitric oxide may be important in preconditioning-induced cardioprotection [45]. Takano and his coworkers demonstrated that on pretreatment with CCPA (A1 receptor agonist) or IB-MECA (A3 receptor agonist), 24 h before 30 min of coronary artery occlusion and 72 h of reperfusion in conscious rabbits reduced the infarct size. However, administration of L-NA (nitric oxide synthase inhibitor) immediately before 30 min of occlusion, selectively abrogated the infarct limiting effect of CCPA, not of IB-MECA, suggesting that A1 receptor activation induced cardioprotective effect is elicited via NOS activation and increased NO production [19]. There have been earlier studies suggesting that adenosine receptor activation increases NO production. Li et al reported that treatment of human iliac arterial endothelial and porcine carotid arterial endothelial cells with CGS-21680 (A2A receptor agonist) increased the levels of NO in the culture medium. Furthermore, pretreatment with CGS-15943 (A1 and A2A receptor antagonist) or ZM-241385 (A2A receptor antagonist) reversed CGS-21680-induced increase in NO levels, suggesting that A2A receptor activation is responsible for an increase in NO production [46]. Vials and Burnstock also reported that adenosine receptor activation increases NO production to produce vasodilation. The authors demonstrated that treatment with CGS-21680 increases the vasodilatory response of NO. Furthermore, prior treatment with L-NAME (nitric oxide inhibitor) inhibited adenosine receptor activation-induced vasodilatory effect of NO [47]. A recent study has shown that adenosine prevents cold-induced injury to the cultured human cardiac microvascular endothelial cells by increasing eNOS phosphorylation at serine 1177 position and NO production and [30]. Another recent study has shown that pharmacological preconditioning with A1 receptor agonist, N6-cyclohexyl adenosine (CHA), led to increase in eNOS phosphorylation and conferred cardioprotection in mice heart. Moreover, there was an increase in the levels of S-nitrosothiols proteins, formed by NO-mediated nitrosylation of proteins. It suggests that adenosine may increase the production of NO to trigger cardioprotection [48].
There have been studies showing that opioid receptors may be involved in adenosine-induced cardioprotection. Surendra et al. demonstrated the functional interaction of δ and κ opioid receptors with adenosine A1 receptors in remote ischemic preconditioning-induced cardioprotection. The plasma dialysate obtained from rabbits after application of remote preconditioning stimulus protected isolated cardiomyocytes from ischemic injury. Furthermore, administration of δ and κ opioid receptor agonists also produced cardioprotection. Administration of A1 receptor antagonist blocked plasma dialysate and opioid receptor activation-induced cardioprotection. Moreover, authors conducted the immunoprecipitation studies to demonstrate the physical molecular association between A1, δ and κ-opioid receptors. Based on these results, it was proposed that the cardioprotective effects of adenosine are mediated through their functional interaction with opioid (particularly δ and κ) receptors [22].
Another study described that adenosine preconditioning-induced cardioprotective effects in ischemia reperfusion injury are mediated through opioid receptors. Administration of adenosine (10 µM) was shown to provide cardioprotection against ischemia reperfusion injury in normal rats. However, at this dose, adenosine failed to protect diabetic rat hearts from ischemic injury. Nevertheless, co-administration of dipyridamole (adenosine reuptake blocker) restored the cardioprotective effects of adenosine preconditioning in diabetic rats. The lack of cardioprotective effects of adenosine in diabetic rats may be possibly due to reduction in adenosine availability. Dipyridamole-mediated increase in adenosine availability may be responsible for restoring the cardioprotective effects of adenosine preconditioning in diabetic rats. Pretreatment with naloxone (opioid receptor antagonist) abolished the cardioprotective effects of adenosine preconditioning in normal rats and adenosine + dipyridamole in diabetic rats suggesting that adenosine preconditioning-mediated cardioprotection is mediated through activation of opioid receptors [49]. Lee et al. described that there is a cross-talk between opioid and adenosine receptors in remifentanil preconditioning-induced cardioprotection. The authors demonstrated that the protective effects of remifentanil were abolished by antagonists of opioid, A1 and A2B receptors suggesting the interrelationship between opioids and adenosine [50]. An earlier study also described the interaction between spinal mu-opioid receptors and adenosine in remote conditioning [51]. A recent study has also shown an inter-relationship between opioids and adenosine in remote preconditioning-induced cardioprotection. In this study, remote preconditioning of trauma was shown to increase the release of adenosine in the spinal cord and administration of mu-opioid receptor antagonist abolished these effects [52].
Inflammation is very well documented to participate in myocardial injury and preconditioning-induced cardioprotection is associated with decrease in inflammation [53]. Adenosine-induced cardioprotection may also be possibly related to decrease in inflammation. A study from our laboratory documented the similarities in remote preconditioning and adenosine preconditioning-induced cardioprotection. Pharmacological preconditioning with adenosine produced cardioprotective effects in a similar manner to remote preconditioning. However, pretreatment with CGRP (calcitonin gene related peptide) release blocker and TRPV (Transient receptor potential vanilloid) inhibitor attenuated remote preconditioning and adenosine preconditioning-induced cardioprotection [16]. TRPV channels are expressed on sensory nerve terminals and these channels participate in releasing CRRP [54]. Therefore, it may be suggested that adenosine produces cardioprotection through activation of TRPV channels, with consequent release of CGRP. It has been demonstrated that TRPV channels inhibits inflammation and apoptosis via release of CGRP during ischemia reperfusion injury in rat hearts [55]. Furthermore, studies from our laboratory have also documented that activation of TRPV channel is important in remote preconditioning-induced cardioprotection [5657].
There have a number of studies showing the anti-inflammatory actions of adenosine preconditioning [5859]. It is reported that adenosine reduces inflammatory response via regulation of A1 receptors and A2 receptors. It was reported that adenosine initially activates pro-inflammatory A1 receptors followed by induction of anti-inflammatory A2B receptors. Moreover, pharmacological preconditioning with CCPA (A1 receptor agonist) was shown to sensitize the A2A receptors and decrease the levels of inflammatory mediators [58]. Nakav et al. also demonstrated that administration of A1 receptor agonists to mice reduces the expression of A1 receptor and induces the expression of A2A receptors on peritoneal mesothelial cells. Furthermore, preconditioning with A1 receptor agonist decreased E. coli-induced increase in the levels of TNF-α, IL-6 and reduced recruitment of leukocytes suggesting anti-inflammatory actions of adenosine preconditioning [59]. A recent study has shown that pretreatment of mice with BAY60-6583 (selective A2B receptor agonist) decreases the recruitment of inflammatory cells in heart to confer cardioprotection against ischemia-reperfusion injury [38].
Kinases serve as important signal transduction pathways in conveying the extracellular signal initiated by preconditioning to inter-cellular targets of cardioprotection. Adenosine preconditioning may activate different kinases to trigger cardioprotection:
Activation of Protein Kinase C (PKC): The studies have shown that adenosine preconditioning may activate PKC signaling pathway to trigger cardioprotection. It has been demonstrated that pharmacological preconditioning of male mice CCPA (adenosine A1 receptor agonist) (100 µg/kg i.v.) induces cardioprotective effects via activation of PKC-δ. CCPA reduced the infarct size, creatine kinase level and improved the left ventricular developed pressure and rate-pressure product in hearts subjected to 40 min of ischemia and 30 min of reperfusion on Langendorff's apparatus. Pretreatment with rottlerin (PKC-δ inhibitor) (50 µg/kg i.p.) abolished the cardioprotective effect of CCPA, revealing that A1 receptor agonist produces its cardioprotective effect via activating PKC-δ. Furthermore, immunoblotting assay showed that PKC-δ was increased after 24 h of CCPA treatment, suggesting that CCPA induces cardioprotective effect via PKC-δ during late phase of pharmacological preconditioning [23]. Dana et al. also demonstrated in rabbits that A1 receptor activation produces cardioprotective effects via activation of PKC. Pretreatment of rabbits with CCPA (100 µg/kg), 24 h before 30 min of regional ischemia and 2 h of reperfusion, reduced myocardial infarct size. Prior administration of chelerythrine chloride (PKC inhibitor) (5 mg/kg) abolished the infarct limiting effect of A1 receptor agonist suggesting that A1 receptor activation produces cardioprotective effect through PKC [24].
Another study has demonstrated that activation of adenosine receptors activate PKC (particularly PKC epsilon) to induce cardioprotection. Adenosine was shown to increase the translocation of PKCε to mitochondria as there was significant increase in PKCε-positive mitochondria in rat cardiomyocytes treated with adenosine. Furthermore, PKC inhibitor (chelerythrine) significantly prevented adenosine-induced increase in PKCε-positive mitochondria [60]. Recently, it has been reported that there is an increase in the levels of adenosine in the spinal cord along with activation of PKCε during remote preconditioning again suggesting the inter-relationship between adenosine and PKCε [52].
Protein Kinase B or Akt: Protein kinase B (also known as Akt) is a serine/threonine-specific protein kinase and it has been shown to play a key role in ischemic preconditioning-induced cardioprotection [61]. Moreover, it is also proposed that adenosine preconditioning also promotes Akt phosphorylation to trigger cardioprotection. Tian et al. reported that administration of BAY60-6583 (selective A2B receptor agonist), before 40 min of ischemia and 60 min of reperfusion, significantly reduced the myocardial infarct size by increasing the levels of phosphorylated form of Akt (p-Akt) and decreasing macrophage and neutrophils infiltration in reperfused hearts. However, pretreatment with ATL-801 (selective A2B receptor antagonist) or wortmannin, a selective phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor, abrogated BAY60-6583-induced increase in p-Akt levels, influx of inflammatory cells and myocardial infarct size. PI3K belongs to the family of intracellular signal transducer enzymes and it may promote phosphorylation of Akt to induce its activation. Therefore, it may be proposed that activation of A2B receptors activates PI3K to promote Akt phosphorylation, which in turn may decrease influx of inflammatory cells to prevent cardiac injury [38]. Recently, it has been reported that pretreatment with N6-cyclohexyladenosine (A1 receptor agonist) significantly increases Akt phosphorylation in mouse heart to trigger cardioprotection against ischemia-reperfusion injury [48].
Activation of Mitogen Activated Protein Kinase (MAPK) and MAPK kinases (MEK): MAP kinases (MAPK) also belong to the family of serine/threonine-specific protein kinase and different members of MAP kinase family include extracellular signal-regulated kinases (ERK), p38 MAP kinases and JNKs (c-Jun N-terminal kinases). Mitogen-activated protein kinase kinase (also known as MEK or MAPKK) is a kinase enzyme which phosphorylates and activates MAP kinase. The studies have the key role of MAP kinases and MEK in ischemic preconditioning-induced cardioprotection [6263]. Scientists have also suggested that adenosine-induced activation of MAP kinase signaling may also be important in inducing cardioprotective effects. Zhao et al. demonstrated that preconditioning with CCPA, A1 receptor agonist (0.1 mg/kg), 24 h before 30 min of ischemia and 30 min of reperfusion on Langendorff's system reduced the post-ischemic infarct size and increased the phosphorylation of p38-MAP kinase. Pretreatment with SB-203580 (p38-MAPK inhibitor) attenuated CCPA-induced phosphorylation of p38-MAPK, suggesting that A1 receptor activation induces p38-MAPK phosphorylation. Furthermore, authors also demonstrated that pretreatment with 5-HD (KATP channel blocker) also inhibited CCPA-induced phosphorylation of p38-MAPK, proposing that A1 receptor activation induces p38-MAPK phosphorylation through KATP channel activation [26]. On the similar lines, Ballard-Croft et al. documented that treatment of rats with AMP-579 (50 µg/kg i.v.) (A1/A2A receptor agonist) reduced the ischemia-reperfusion induced increase in infarct size and increased the phosphorylation of p38-MAP kinase in nuclear fractions. However, pretreatment with SB-203580 (p38-MAPK inhibitor) abolished the p38-MAP kinase phosphorylation and infarct limiting effect of AMP-579, suggesting that A1/A2A receptor activation induces p38-MAPK phosphorylation to confer cardioprotection [27]. Another study also reports that preconditioning with AMP579 (A1 and A2B receptor agonist) attenuates myocardial stunning in pigs through activation of p38 MAP kinase [64]. An earlier study of Dana et al. also reported an increase in p38-MAPK activity following treatment with CCPA. However, pretreatment with chelerythrine chloride (PKC inhibitor) or lavendustin A (tyrosine kinase inhibitor) abrogated CPA-induced increase in p38-MAP kinase activity. It suggests that adenosine A1 receptor activation may increase p38-MAP kinase activity, possibly through modulation of PKC and tyrosine kinase pathway [24].
Germack and Dickenson reported that preconditioning of neonatal rat cardiomyocytes with CPA (A1 receptor agonist) and CI-IB-MECA (A3 receptor agonist) protects ischemia-reperfusion injury by activating MEK 1/ERK signaling pathway. Pretreatment with MEK 1 inhibitor (PD 98059) was shown to suppress the cardioprotective effects of adenosine agonists, suggesting that adenosine induces cardioprotection via activation of MEK 1 pathway. Furthermore, treatment with CPA and CI-IB-MECA enhanced phosphorylation of ERK 1/2, suggesting that A1 and A3 receptors-induced activation of MEK 1 phosphorylates ERK 1/2 to trigger cardioprotection [25]. Another study demonstrated that preconditioning effects of AMP-579 (A1/A2 receptor agonist) and CGS-21680 (A2A receptor agonist) on rabbit hearts was abrogated in the presence in PD 98059 (MEK 1 inhibitor) suggesting that adenosine confers cardioprotection via activating MEK pathway [65]. William-Pritchard et al. demonstrated that adenosine preconditioning-induced increase in phosphorylation of ERK 1/2 is dependent of activation of EGFR (epidermal growth factor receptor) and MMP (matrix metalloproteinase). It was shown that CCPA (A1 receptor agonist)-induced cardioprotective effects and ERK 1/2 phosphorylation are abolished in the presence of EGFR inhibitor (AG 1478) or MMP inhibitor (GM 6001) confirming that adenosine may activate EGFR and MMP to increase ERK 1/2 phosphorylation and confer cardioprotection [28].
Go to : 
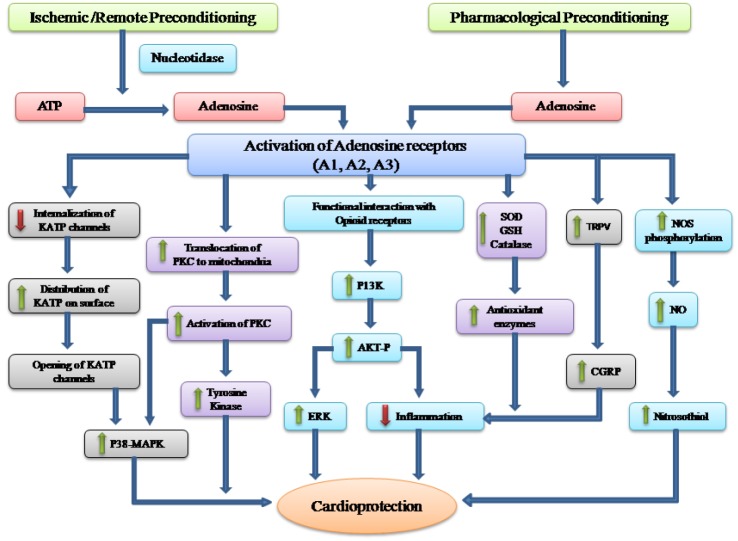
Ischemic, remote and pharmacological preconditioning are powerful tools to attenuate myocardial ischemic injury and there have been a number of studies documenting the key role of adenosine in ischemic [29] and remote preconditioning-induced cardioprotection [15]. During preconditioning stimulus, ATP is hydrolyzed in the presence of nucleotidase to give rise to adenosine, which acts on different adenosine receptors viz . A1, A2A, A2B and A3 located on different portions of heart. However, majority of studies have documented the more predominant role of A1 and A3 receptors in mediating cardioprotective effects of adenosine [1718]. Activation of adenosine may activate plethora of signaling cascade, which may either independently or in association with one another may confer cardioprotection against ischemia reperfusion injury (Fig. 1). Activation of adenosine receptors may preserve the expression of KATP channels on the surface by attenuating ischemia-induced decrease in sarcolemmal KATP channel expression and internalization of KATP channels to endosomal compartments [42]. The opening of KATP channels may trigger a number of cardioprotective pathways including activation of p38 MAP kinase [26]. Adenosine-induced increase in translocation of PKC to mitochondria with subsequent activation of PKC may also be critical mechanism in activating p38 MAP kinase [24]. p38 MAP kinase is a crucial pro-survival kinase and its activation imparts cardioprotection during different forms of preconditioning [2766].
Various studies have documented the functional and physical interaction between adenosine and opioid receptors. Indeed, there is a cross-talk between these receptors in inducing cardioprotective effects [505152]. It has been documented that adenosine may activate opioid receptors to induce cardioprotection during preconditioning [2249]. It is also possible that opioid receptors activate PI3K enzyme and increase Akt phosphorylation, which subsequently causes activation of ERK 1/2 to trigger cardioprotection [67]. Adenosine preconditioning-induced activation of PI3K-Akt pathway is very important in reducing recruitment of inflammatory cells in heart during ischemia-reperfusion phase [38]. Moreover, adenosine may also increase the levels of antioxidant enzymes including SOD, catalase, and glutathione peroxidase to decrease the burden of oxidative stress during ischemia reperfusion [43]. Adenosine-induced increase in anti-oxidant enzymes may also help in attenuating inflammation-induced myocardial injury. The study from our laboratory suggests that adenosine preconditioning or remote preconditioning stimulus may activate TRPV channels, which may subsequently release CGRP to confer cardioprotection [16]. It is well documented that activation of TRPV and release of CGRP during preconditioning phase may reduce inflammatory reactions during sustained ischemiareperfusion phase [55]. Furthermore, adenosine preconditioning may trigger NOS phosphorylation to increase its activity, which subsequently increases NO production [30]. NO may increase nitrosylation of proteins to increase the levels of S-nitrosothiols, which helps in attenuating myocardial injury during ischemia reperfusion [48].
Go to : 
Adenosine preconditioning is a powerful tool in inducing cardioprotection and adenosine may predominantly activate A1 and A3 receptors to trigger different cardioprotective signals. These pathways include increase in opening of KATP channels and PKC activation to increase p38MAP kinase activity; activation of opioid receptors to activate PI3K-Akt pathway to decrease inflammation and activate ERK 1/2; activation of TRPV to release CGRP to decrease inflammation; increase in antioxidant enzymes and increase in NO production to increase the levels of S-nitrosothiols.
Go to : 
ACKNOWLEDGEMENTS
The authors are thankful to Department of Science and Technology F. No. SB/SO/HS/0004/2013, New Delhi for their gratefulness for providing us financial assistance and Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India for supporting us.
Go to : 
Notes
Author contributions: L.S. did literature survey and wrote the manuscript. R.K. was involved in revising the manuscript. N.S. helpled in writing manuscript. A.S.J. conceived the idea, designed the manuscript and performed editing of manuscript.
Go to : 
References
1. Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V. Adenosine receptors: expression, function and regulation. Int J Mol Sci. 2014; 15:2024–2052. PMID: 24477263.

2. Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009; 193:161–188.

3. Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014; 7:581–591. PMID: 24835328.
4. Liang BT, Jacobson KA. Adenosine and ischemic preconditioning. Curr Pharm Des. 1999; 5:1029–1041. PMID: 10607860.
5. Headrick JP, Ashton KJ, Rose'meyer RB, Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacol Ther. 2013; 140:92–111. PMID: 23764371.

6. Ohta A, Sitkovsky M. The adenosinergic immunomodulatory drugs. Curr Opin Pharmacol. 2009; 9:501–506. PMID: 19539527.

7. Sachdeva S, Gupta M. Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J. 2013; 21:245–253. PMID: 23960840.

8. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74:1124–1136. PMID: 3769170.

9. McCully JD, Toyoda Y, Uematsu M, Stewart RD, Levitsky S. Adenosine-enhanced ischemic preconditioning: adenosine receptor involvement during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2001; 280:H591–H602. PMID: 11158956.

10. de Jong JW, de Jonge R, Keijzer E, Bradamante S. The role of adenosine in preconditioning. Pharmacol Ther. 2000; 87:141–149. PMID: 11007996.

11. Kitakaze M, Hori M, Takashima S, Sato H, Inoue M, Kamada T. Ischemic preconditioning increases adenosine release and 5′-nucleotidase activity during myocardial ischemia and reperfusion in dogs. Implications for myocardial salvage. Circulation. 1993; 87:208–215. PMID: 8419009.

12. Hori M, Kitakaze M. Adenosine, the heart, and coronary circulation. Hypertension. 1991; 18:565–574. PMID: 1937658.

13. Schulz R, Rose J, Post H, Heusch G. Involvement of endogenous adenosine in ischaemic preconditioning in swine. Pflugers Arch. 1995; 430:273–282. PMID: 7675637.

14. Randhawa PK, Bali A, Jaggi AS. RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. Eur J Pharmacol. 2015; 746:317–332. PMID: 25176179.

15. Randhawa PK, Jaggi AS. Unraveling the role of adenosine in remote ischemic preconditioning-induced cardioprotection. Life Sci. 2016; 155:140–114. PMID: 27157518.

16. Singh A, Randhawa PK, Bali A, Singh N, Jaggi AS. Exploring the role of TRPV and CGRP in adenosine preconditioning and remote hind limb preconditioning-induced cardioprotection in rats. Cardiovasc Drugs Ther. 2017; 31:133–143. PMID: 28194544.

17. Baxter GF, Yellon DM. ATP-sensitive K+ channels mediate the delayed cardioprotective effect of adenosine A1 receptor activation. J Mol Cell Cardiol. 1999; 31:981–989. PMID: 10336838.
18. Tracey WR, Magee W, Masamune H, Oleynek JJ, Hill RJ. Selective activation of adenosine A3 receptors with N6-(3-chlorobenzyl)-5′-N-methylcarboxamidoadenosine (CB-MECA) provides cardioprotection via KATP channel activation. Cardiovasc Res. 1998; 40:138–145. PMID: 9876326.

19. Takano H, Bolli R, Black RG Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001; 88:520–528. PMID: 11249876.
20. Dana A, Jonassen AK, Yamashita N, Yellon DM. Adenosine A(1) receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation. 2000; 101:2841–2848. PMID: 10859291.
21. Hochhauser E, Kaminski O, Shalom H, Leshem D, Shneyvays V, Shainberg A, Vidne BA. Role of adenosine receptor activation in antioxidant enzyme regulation during ischemia-reperfusion in the isolated rat heart. Antioxid Redox Signal. 2004; 6:335–344. PMID: 15025935.

22. Surendra H, Diaz RJ, Harvey K, Tropak M, Callahan J, Hinek A, Hossain T, Redington A, Wilson GJ. Interaction of δ and κ opioid receptors with adenosine A1 receptors mediates cardioprotection by remote ischemic preconditioning. J Mol Cell Cardiol. 2013; 60:142–150. PMID: 23608604.

23. Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am J Physiol Heart Circ Physiol. 2002; 283:H296–H301. PMID: 12063302.
24. Dana A, Skarli M, Papakrivopoulou J, Yellon DM. Adenosine A(1) receptor induced delayed preconditioning in rabbits: induction of p38 mitogen-activated protein kinase activation and Hsp27 phosphorylation via a tyrosine kinase- and protein kinase C-dependent mechanism. Circ Res. 2000; 86:989–997. PMID: 10807872.
25. Germack R, Dickenson JM. Adenosine triggers preconditioning through MEK/ERK1/2 signalling pathway during hypoxia/ reoxygenation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2005; 39:429–442. PMID: 16005018.
26. Zhao TC, Hines DS, Kukreja RC. Adenosine-induced late preconditioning in mouse hearts: role of p38 MAP kinase and mitochondrial K(ATP) channels. Am J Physiol Heart Circ Physiol. 2001; 280:H1278–H1285. PMID: 11179074.

27. Ballard-Croft C, Kristo G, Yoshimura Y, Reid E, Keith BJ, Mentzer RM Jr, Lasley RD. Acute adenosine preconditioning is mediated by p38 MAPK activation in discrete subcellular compartments. Am J Physiol Heart Circ Physiol. 2005; 288:H1359–H1366. PMID: 15539417.

28. Williams-Pritchard G, Knight M, Hoe LS, Headrick JP, Peart JN. Essential role of EGFR in cardioprotection and signaling responses to A1 adenosine receptors and ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2011; 300:H2161–H2168. PMID: 21460200.

29. Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol. 2000; 62:79–109. PMID: 10845085.

30. Li Y, Hu XX, Fu L, Chen J, Lu LH, Liu X, Xu Z, Zhou L, Wang ZP, Zhang X, Ou ZJ, Ou JS. Time window is important for adenosine preventing cold-induced injury to the endothelium. J Cardiovasc Pharmacol. 2017; 69:382–388. PMID: 28581447.

31. Wolff G, Truse R, Decking U. Extracellular adenosine formation by ecto-5′-nucleotidase (CD73) is no essential trigger for early phase ischemic preconditioning. PLoS One. 2015; 10:e0135086. PMID: 26261991.

32. Mastitskaya S, Marina N, Gourine A, Gilbey MP, Spyer KM, Teschemacher AG, Kasparov S, Trapp S, Ackland GL, Gourine AV. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012; 95:487–494. PMID: 22739118.

33. Jammes Y, Joulia F, Steinberg JG, Ravailhe S, Delpierre S, Condo J, Guieu R, Delliaux S. Endogenous adenosine release is involved in the control of heart rate in rats. Can J Physiol Pharmacol. 2015; 93:667–675. PMID: 26222197.

34. Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998; 275:H1542–H1547. PMID: 9815059.
35. Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002; 283:H29–H37. PMID: 12063271.
36. Schulte G, Sommerschild H, Yang J, Tokuno S, Goiny M, Lövdahl C, Johansson B, Fredholm BB, Valen G. Adenosine A receptors are necessary for protection of the murine heart by remote, delayed adaptation to ischaemia. Acta Physiol Scand. 2004; 182:133–143. PMID: 15450109.
37. Leung CH, Wang L, Nielsen JM, Tropak MB, Fu YY, Kato H, Callahan J, Redington AN, Caldarone CA. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014; 28:7–17. PMID: 24018748.

38. Tian Y, Piras BA, Kron IL, French BA, Yang Z. Adenosine 2B receptor activation reduces myocardial reperfusion injury by promoting anti-inflammatory macrophages differentiation via PI3K/Akt pathway. Oxid Med Cell Longev. 2015; 2015:585297. PMID: 26161239.

39. Montero MF, Saurim R, Bonservizi WG, Koike MK, Taha MO. Heart injury following intestinal ischemia reperfusion in rats is attenuated by association of ischemic preconditioning and adenosine. Acta Cir Bras. 2014; 29(Suppl 2):67–71. PMID: 25229518.

40. Shehata M. Cardioprotective effects of intracoronary adenosine in diabetic patients undergoing elective percutaneous coronary intervention. Minerva Cardioangiol. 2014; 62:461–471. PMID: 24699551.
41. Gopalakrishnan SM, Buckner SA, Milicic I, Groebe DR, Whiteaker KL, Burns DJ, Warrior U, Gopalakrishnan M. Functional characterization of adenosine receptors and coupling to ATP-sensitive K+ channels in Guinea pig urinary bladder smooth muscle. J Pharmacol Exp Ther. 2002; 300:910–917. PMID: 11861797.
42. Yang HQ, Foster MN, Jana K, Ho J, Rindler MJ, Coetzee WA. Plasticity of sarcolemmal KATP channel surface expression: relevance during ischemia and ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2016; 310:H1558–H1566. PMID: 27037371.

43. Husain K, Somani SM. Interaction of exercise and adenosine receptor agonist and antagonist on rat heart antioxidant defense system. Mol Cell Biochem. 2005; 270:209–214. PMID: 15792369.

44. Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem Biophys Res Commun. 1994; 201:508–515. PMID: 8002980.

45. Aggarwal S, Randhawa PK, Singh N, Jaggi AS. Preconditioning at a distance: Involvement of endothelial vasoactive substances in cardioprotection against ischemia-reperfusion injury. Life Sci. 2016; 151:250–258. PMID: 26979771.

46. Li Jm, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, Dobson JG Jr. Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res. 1998; 80:357–364. PMID: 9878338.
47. Vials A, Burnstock G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br J Pharmacol. 1993; 109:424–429. PMID: 8358543.

48. Shao Q, Casin KM, Mackowski N, Murphy E, Steenbergen C, Kohr MJ. Adenosine A1 receptor activation increases myocardial protein S-nitrosothiols and elicits protection from ischemia-reperfusion injury in male and female hearts. PLoS One. 2017; 12:e0177315. PMID: 28493997.

49. Sharma NK, Mahadevan N, Balakumar P. Adenosine transport blockade restores attenuated cardioprotective effects of adenosine preconditioning in the isolated diabetic rat heart: potential crosstalk with opioid receptors. Cardiovasc Toxicol. 2013; 13:22–32. PMID: 22948709.

50. Lee YC, Jung J, Park SJ. Remifentanil-induced preconditioning has cross-talk with A1 and A2B adenosine receptors in ischemicreperfused rat heart. Bosn J Basic Med Sci. 2016; 16:64–70. PMID: 26773185.

51. Yao L, Wong GT, Xia Z, Irwin MG. Interaction between spinal opioid and adenosine receptors in remote cardiac preconditioning: effect of intrathecal morphine. J Cardiothorac Vasc Anesth. 2011; 25:444–448. PMID: 20688538.

52. Mei B, Li W, Cheng X, Liu X, Gu E, Zhang Y. Activating mu-opioid receptors in the spinal cord mediates the cardioprotective effect of remote preconditioning of trauma. Cardiol J. 2017; 24:314–323. PMID: 27586455.

53. Avraamidou A, Marinis A, Asonitis S, Perrea D, Polymeneas G, Voros D, Argyra E. The impact of ischemic preconditioning on hemodynamic, biochemical and inflammatory alterations induced by intra-abdominal hypertension: an experimental study in a porcine model. Langenbecks Arch Surg. 2012; 397:1333–1341. PMID: 22760999.

54. Randhawa PK, Jaggi AS. TRPV1 and TRPV4 channels: potential therapeutic targets for ischemic conditioning-induced cardioprotection. Eur J Pharmacol. 2015; 746:180–185. PMID: 25449039.

55. Lei J, Zhu F, Zhang Y, Duan L, Lei H, Huang W. Transient receptor potential vanilloid subtype 1 inhibits inflammation and apoptosis via the release of calcitonin gene-related peptide in the heart after myocardial infarction. Cardiology. 2016; 134:436–443. PMID: 27144592.

56. Randhawa PK, Jaggi AS. Investigating the involvement of glycogen synthase kinase-3β and gap junction signaling in TRPV1 and remote hind preconditioning-induced cardioprotection. Eur J Pharmacol. 2017; 814:9–17. PMID: 28755986.

57. Randhawa PK, Jaggi AS. Investigating the involvement of TRPV1 ion channels in remote hind limb preconditioning-induced cardioprotection in rats. Naunyn Schmiedebergs Arch Pharmacol. 2017; 390:117–126. PMID: 27752734.

58. Naamani O, Chaimovitz C, Douvdevani A. Pharmacological preconditioning with adenosine A(1) receptor agonist suppresses cellular immune response by an A(2A) receptor dependent mechanism. Int Immunopharmacol. 2014; 20:205–212. PMID: 24560904.

59. Nakav S, Chaimovitz C, Sufaro Y, Lewis EC, Shaked G, Czeiger D, Zlotnik M, Douvdevani A. Anti-inflammatory preconditioning by agonists of adenosine A1 receptor. PLoS One. 2008; 3:e2107. PMID: 18461129.

60. Yang Z, Sun W, Hu K. Molecular mechanism underlying adenosine receptor-mediated mitochondrial targeting of protein kinase C. Biochim Biophys Acta. 2012; 1823:950–958. PMID: 22233927.

61. Lai CC, Tang CY, Chiang SC, Tseng KW, Huang CH. Ischemic preconditioning activates prosurvival kinases and reduces myocardial apoptosis. J Chin Med Assoc. 2015; 78:460–468. PMID: 26071976.

62. da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology. 2004; 100:59–69. PMID: 14695725.

63. Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005; 112:1971–1978. PMID: 16172266.
64. Yoshimura Y, Kristo G, Keith BJ, Jahania SA, Mentzer RM Jr, Lasley RD. The p38 MAPK inhibitor SB203580 blocks adenosine A(1) receptor-induced attenuation of in vivo myocardial stunning. Cardiovasc Drugs Ther. 2004; 18:433–440. PMID: 15770430.

65. Kis A, Baxter GF, Yellon DM. Limitation of myocardial reperfusion injury by AMP579, an adenosine A1/A2A receptor agonist: role of A2A receptor and Erk1/2. Cardiovasc Drugs Ther. 2003; 17:415–425. PMID: 15107596.
66. Yoshino T, Nagoshi T, Anzawa R, Kashiwagi Y, Ito K, Katoh D, Fujisaki M, Kayama Y, Date T, Hongo K, Yoshimura M. Preconditioning actions of aldosterone through p38 signaling modulation in isolated rat hearts. J Endocrinol. 2014; 222:289–299. PMID: 24895416.

67. Dou MY, Wu H, Zhu HJ, Jin SY, Zhang Y, He SF. Remifentanil preconditioning protects rat cardiomyocytes against hypoxia-reoxygenation injury via δ-opioid receptor mediated activation of PI3K/ Akt and ERK pathways. Eur J Pharmacol. 2016; 789:395–401. PMID: 27492364.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download