INTRODUCTION
METHODS
Animals
Drug treatment
Chronic unpredictable stress (CUS) procedure
Forced swim test (FST)
Locomotor activity test (LMA)
Novelty suppressed feeding test (NSFT)
Chromatin immunoprecipitation (ChIP) assay
Quantitative real-time PCR
Western blot analysis
Virus production and purification
Stereotaxic surgery and infusions
Immunohistochemistry
Statistical analysis
RESULTS
CPP rapidly induced HDAC5 phosphorylation in a time-dependent manner and triggered cytoplasmic localization of HDAC5 in the rat hippocampus
 | Fig. 1CPP rapidly induced HDAC5 phosphorylation in a time-dependent manner and triggered cytoplasmic localization of HDAC5 in the rat hippocampus.(A) CPP (0.5 mg/kg) increased phosphorylation of HDAC5 in the rat hippocampus in a time-dependent manner. Peak phosphorylation was observed at approximately 0.5 h after treatment. (B) Quantitative analysis of the HDAC5 phosphorylation shown in (A) (n=3 per group). (C) CPP (0.5 mg/kg) induced phosphorylation of cytoplasmic HDAC5 at 1 h post-treatment. (D) Quantitative analysis of the HDAC5 phosphorylation in cytoplasm shown in (C) (n=7 per group). Values are expressed as mean±S.E.M. Student's t-test, *p<0.05, **p<0.01, ***p<0.001 compared to the CTL group.
|
CPP increased phosphorylation of PKD and CaMKII and upregulated BDNF expression
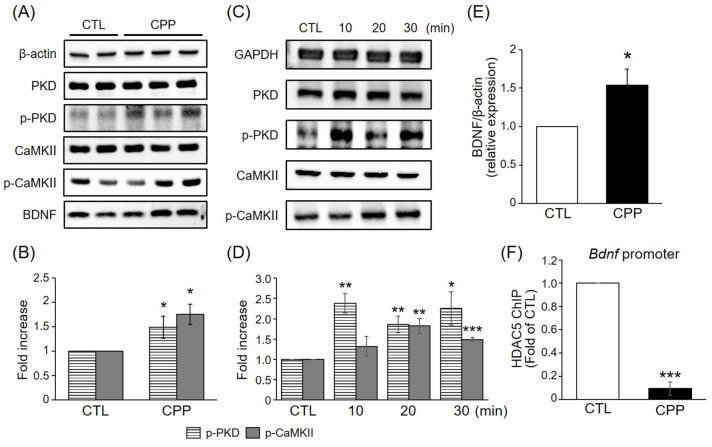 | Fig. 2CPP increased phosphorylation of PKD and CaMKII and upregulated BDNF expression.(A) Representative immunoblots of hippocampal extracts of rats exposed to CPP (0.5 mg/kg). Extracts were generated 1 h after treatment. Protein levels were normalized to that of β-actin. (B) Quantitative analysis of p-PKD and p-CaMKII levels. Values were normalized to that of total PKD or CaMKII and expressed relative to CTL (n=6 per group). (C) CPP (0.5 mg/kg) increased phosphorylation of PKD and CaMKII in the rat hippocampus in a time-dependent manner. Peak phosphorylation of PKD and CaMKII was observed at approximately 10 min and 20 min respectively after treatment. (D) Quantitative analysis of the PKD and CaMKII phosphorylation is shown in (C) (n=3 per group). (E) Quantitative analysis of BDNF levels shown in (A). Values were normalized to that of β-actin (n=6 per group). (F) ChIP assays. Decreased binding of HDAC5 to the Bdnf promoter in the hippocampus of rats treated with CPP (0.5 mg/kg) (n=3 per group). Values are expressed as mean±S.E.M. Student's t-test, *p<0.05, **p<0.01, ***p<0.001 compared to the CTL group.
|
CPP had a rapid antidepressant-like effect in the FST and the NSFT
 | Fig. 3CPP had a rapid antidepressant-like effect in the FST and the NSFT.(A) Schematic diagram of the experimental design. Rats were subjected to chronic unpredictable stress (CUS) for 28 days. CPP (0.5 mg/kg) was administered 1 h before the FST and NSFT. (B) The mean body weight of stressed rats was lower than that of nonstressed rats (Nonstressed, n=17; CUS, n=15). Values are expressed as mean±S.E.M. Student's t-test, ***p<0.001 compared to the CTL group. (C) Immobility times in the nonstressed and CUS groups at the early stage of the FST (5 min). Main effect of CPP: F1,28=13.637, p<0.01; main effect of stress: F1,28=0.024, p>0.05; interaction: F1,28=0.56, p>0.05. (D) Immobility times in the nonstressed and CUS groups at the late stage of the FST (10 min). Main effect of CPP: F1,28=6.939, p<0.05; main effect of stress: F1,28=0.526, p>0.05; interaction: F1,28=3.218, p>0.05 (Nonstressed CTL, n=9; Nonstressed CPP, n=8; CUS CTL, n=7; CUS CPP, n=8). (E) NSFT. Main effect of CPP: F1,16=4.476, p=0.05; main effect of stress: F1,16=1.119, p>0.05; interaction: F1,16=3.841, p>0.05 (Nonstressed CTL, n=5; Nonstressed CPP, n=5; CUS CTL, n=5; CUS CPP, n=5). (F) Home cage feeding test. Main effect of CPP: F1,16=0.008, p>0.05; main effect of stress: F1,16=0.006, p>0.05; interaction: F1,16=0.239, p>0.05 (n=5 per group). (G) Locomotor activity. Main effect of CPP: F1,16=0.393, p>0.05; main effect of stress: F1,16=3.038, p>0.05; interaction: F1,16=0.132, p>0.05 (n=5 per group). Values are expressed as mean±S.E.M. Two-way ANOVA was followed by LSD post hoc analysis. *p<0.05, ***p<0.001 compared to nonstressed control rats. #p<0.05, ##p<0.01 compared to CUS control rats.
|
HDAC5 knockdown produced antidepressant effects and occluded the actions of CPP in chronically stressed rats
 | Fig. 4HDAC5 knockdown produced antidepressant effects and occluded the actions of CPP in chronically stressed rats.(A) Schematic diagram of the experimental design. Rats were injected bilaterally with lenti-GFP or lenti-shHDAC5. (B) GFP expression in the DG (scale bar, 400 µm). (C, D) Efficiency of lentiviral-mediated knockdown of HDAC5 in the DG on the protein and mRNA levels (n=3 per group). (E) Locomotor activity, as assessed by total distance moved in the box (n=15 per group). Values are expressed as mean±S.E.M. Student's t-test. (F) Immobility times in the lenti-GFP and lenti-shHDAC5 groups at the early stage of the FST (5 min). Main effect of CPP: F1,27=5.006, p<0.05; main effect of virus: F1,27=11.678, p<0.01; interaction: F1,27=3.979, p>0.05. (G) Immobility times in the lenti-GFP and lenti-shHDAC5 groups at the late stage of the FST (10 min). Main effect of CPP: F1,27=5.496, p<0.05; main effect of virus: F1,27=9.851, p<0.01; interaction: F1,27=2.379, p>0.05. (Lenti-GFP CTL, n=7; Lenti-GFP CPP, Lenti-shHDAC5-GFP CTL, Lenti-shHDAC5-GFP CPP: n=8). (H) Bdnf mRNA level in the lenti-GFP and lenti-shHDAC5 groups (n=3 per group). Main effect of CPP: F1,12=0.582, p>0.05; main effect of virus: F1,27=13.769, p<0.01; interaction: F1,27=2.657, p>0.05. (I) BDNF protein level in the lenti-GFP and lenti-shHDAC5 groups (n=3 per group). Main effect of CPP: F1,8=5.884, p<0.05; main effect of virus: F1,8=1.278, p>0.05; interaction: F1,8=4.991, p>0.05. Two-way ANOVA was followed by LSD post hoc analysis. *p<0.05, **p<0.01 compared to lenti-GFP-CTL rats. n.s., not significant.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download