1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385:117–171. PMID:
25530442.
2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017; 135:e146–e603. PMID:
28122885.

3. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587. PMID:
7477192.
4. Lambrinos A, Schaink AK, Dhalla I, Krings T, Casaubon LK, Sikich N, Lum C, Bharatha A, Pereira VM, Stotts G, Saposnik G, Kelloway L, Xie X, Hill MD. Mechanical thrombectomy in acute ischemic stroke: a systematic review. Can J Neurol Sci. 2016; 43:455–460. PMID:
27071728.

5. Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010; 24:708–723. PMID:
19770034.

6. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009; 7:97. PMID:
19919699.

7. Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Postischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 2009; 276:13–26. PMID:
19087196.

8. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003; 183:25–33. PMID:
12957485.

9. Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003; 117:531–539. PMID:
12617960.

10. Gerhard A, Neumaier B, Elitok E, Glatting G, Ries V, Tomczak R, Ludolph AC, Reske SN. In vivo imaging of activated microglia using [11C]PK11195 and positron emission tomography in patients after ischemic stroke. Neuroreport. 2000; 11:2957–2960. PMID:
11006973.
11. Price CJ, Menon DK, Peters AM, Ballinger JR, Barber RW, Balan KK, Lynch A, Xuereb JH, Fryer T, Guadagno JV, Warburton EA. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004; 35:1659–1664. PMID:
15155970.
12. Agata I1. Studies on caffeic acid derivatives in medicinal plants. Yakugaku Zasshi. 1999; 119:237–248. PMID:
10228448.

13. Sud'ina GF, Mirzoeva OK, Pushkareva MA, Korshunova GA, Sumbatyan NV, Varfolomeev SD. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993; 329:21–24. PMID:
7689063.
14. Altuğ ME, Serarslan Y, Bal R, Kontaş T, Ekici F, Melek IM, Aslan H, Duman T. Caffeic acid phenethyl ester protects rabbit brains against permanent focal ischemia by antioxidant action: a biochemical and planimetric study. Brain Res. 2008; 1201:135–142. PMID:
18308295.

15. Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz M, Oztas E. Protective effects of caffeic acid phenethyl ester against experimental allergic encephalomyelitis-induced oxidative stress in rats. Free Radic Biol Med. 2004; 37:386–394. PMID:
15223072.

16. Khan M, Elango C, Ansari MA, Singh I, Singh AK. Caffeic acid phenethyl ester reduces neurovascular inflammation and protects rat brain following transient focal cerebral ischemia. J Neurochem. 2007; 102:365–377. PMID:
17437550.

17. Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SY, Oh HY. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol. 2004; 4:429–436. PMID:
15037220.

18. Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007; 113:1–14. PMID:
17580109.

19. Shin TK, Kang MS, Lee HY, Seo MS, Kim SG, Kim CD, Lee WS. Fluoxetine and sertraline attenuate postischemic brain injury in mice. Korean J Physiol Pharmacol. 2009; 13:257–263. PMID:
19885045.

20. Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986; 17:1304–1308. PMID:
2433817.

21. Park CH, Shin TK, Lee HY, Kim SJ, Lee WS. Matrix metalloproteinase inhibitors attenuate neuroinflammation following focal cerebral ischemia in mice. Korean J Physiol Pharmacol. 2011; 15:115–122. PMID:
21660152.

22. Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002; 73(Suppl 1):S1–S6. PMID:
12495704.

23. Ilhan A, Koltuksuz U, Ozen S, Uz E, Ciralik H, Akyol O. The effects of caffeic acid phenethyl ester (CAPE) on spinal cord ischemia/reperfusion injury in rabbits. Eur J Cardiothorac Surg. 1999; 16:458–463. PMID:
10571095.

24. Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol. 1994; 48:595–608. PMID:
7520698.

25. Lee SK, Song L, Mata-Greenwood E, Kelloff GJ, Steele VE, Pezzuto JM. Modulation of
in vitro biomarkers of the carcinogenic process by chemopreventive agents. Anticancer Res. 1999; 19:35–44. PMID:
10226522.
26. Uz E, Söğüt S, Sahin S, Var A, Ozyurt H, Güleç M, Akyol O. The protective role of caffeic acid phenethyl ester (CAPE) on testicular tissue after testicular torsion and detorsion. World J Urol. 2002; 20:264–270. PMID:
12215859.

27. Mohamadin AM, Hammad LN, El-Bab MF, Abdel Gawad HS. Attenuation of oxidative stress in plasma and tissues of rats with experimentally induced hyperthyroidism by caffeic acid phenylethyl ester. Basic Clin Pharmacol Toxicol. 2007; 100:84–90. PMID:
17244256.

28. Grome JJ, Gojowczyk G, Hofmann W, Graham DI. Quantitation of photochemically induced focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1988; 8:89–95. PMID:
3339108.

29. Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006; 26:1057–1083. PMID:
16710759.

30. Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981; 12:723–725. PMID:
6272455.

31. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999; 22:391–397. PMID:
10441299.

32. Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013; 2013:746068. PMID:
24223607.

33. Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013; 6:834–851. PMID:
24006091.

34. Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007; 4:12. PMID:
17474992.

35. Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-a expression in ischemic neurons. Stroke. 1994; 25:1481–1488. PMID:
8023366.
36. Zaremba J, Losy J. Early TNF-α levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001; 104:288–295. PMID:
11696023.

37. Sriram K, O'Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007; 2:140–153. PMID:
18040839.
38. Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow C. Brain-specific knock-out of hypoxia-inducible factor-1α reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005; 25:4099–4107. PMID:
15843612.

39. Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, Nanda A, Zhou C. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 2007; 102:1831–1841. PMID:
17532791.
40. Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001; 21:1105–1114. PMID:
11524615.

41. Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003; 14:524–534. PMID:
14678768.
42. Sharp FR, Bergeron M, Bernaudin M. Hypoxia-inducible factor in brain. Adv Exp Med Biol. 2001; 502:273–291. PMID:
11950144.

43. Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1α mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999; 19:6818–6824. PMID:
10436039.

44. Chen C, Hu Q, Yan J, Yang X, Shi X, Lei J, Chen L, Huang H, Han J, Zhang JH, Zhou C. Early inhibition of HIF-1α with small interfering RNA reduces ischemic-reperfused brain injury in rats. Neurobiol Dis. 2009; 33:509–517. PMID:
19166937.

45. Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997; 272:5375–5381. PMID:
9038135.

46. Dawn B, Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 2005; 289:H522–H524. PMID:
16014614.

47. Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999; 72:1187–1203. PMID:
10037492.

48. Fu R, Zhao ZQ, Zhao HY, Zhao JS, Zhu XL. Expression of heme oxygenase-1 protein and messenger RNA in permanent cerebral ischemia in rats. Neurol Res. 2006; 28:38–45. PMID:
16464361.

49. Demougeot C, Van Hoecke M, Bertrand N, Prigent-Tessier A, Mossiat C, Beley A, Marie C. Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2′-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharmacol Exp Ther. 2004; 311:1080–1087. PMID:
15280435.

50. Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001; 902:171–177. PMID:
11384610.

51. Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002; 22:308–317. PMID:
11891436.

52. Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NE, Vogel SN. Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab. 2003; 23:748–755. PMID:
12796723.

53. Konsman JP, Blond D, Vigues S. Neurobiology of interleukin-1 receptors: getting the message. Eur Cytokine Netw. 2000; 11:699–702. PMID:
11125316.
54. Harkness KA, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003; 142:1–9. PMID:
14512159.

55. Pérez-De La Cruz V, Königsberg M, Santamaría A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets. 2007; 6:398–410. PMID:
18220779.
56. Saito K, Nowak TS Jr, Markey SP, Heyes MP. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J Neurochem. 1993; 60:180–192. PMID:
8417138.

57. Saito K, Nowak TS Jr, Suyama K, Quearry BJ, Saito M, Crowley JS, Markey SP, Heyes MP. Kynurenine pathway enzymes in brain: responses to ischemic brain injury versus systemic immune activation. J Neurochem. 1993; 61:2061–2070. PMID:
8245962.

58. Spalletta G, Bossù P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006; 11:984–991. PMID:
16894392.

59. Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999; 100:203–215. PMID:
10695731.

60. Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000; 23:618–625. PMID:
11137152.

61. Luheshi NM, Kovács KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation. 2011; 8:186. PMID:
22206506.

62. Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002; 33:2115–2122. PMID:
12154274.

63. Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, Arakawa S, Sugimori H, Kamouchi M, Kitazono T, Iida M. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005; 111:913–919. PMID:
15710762.

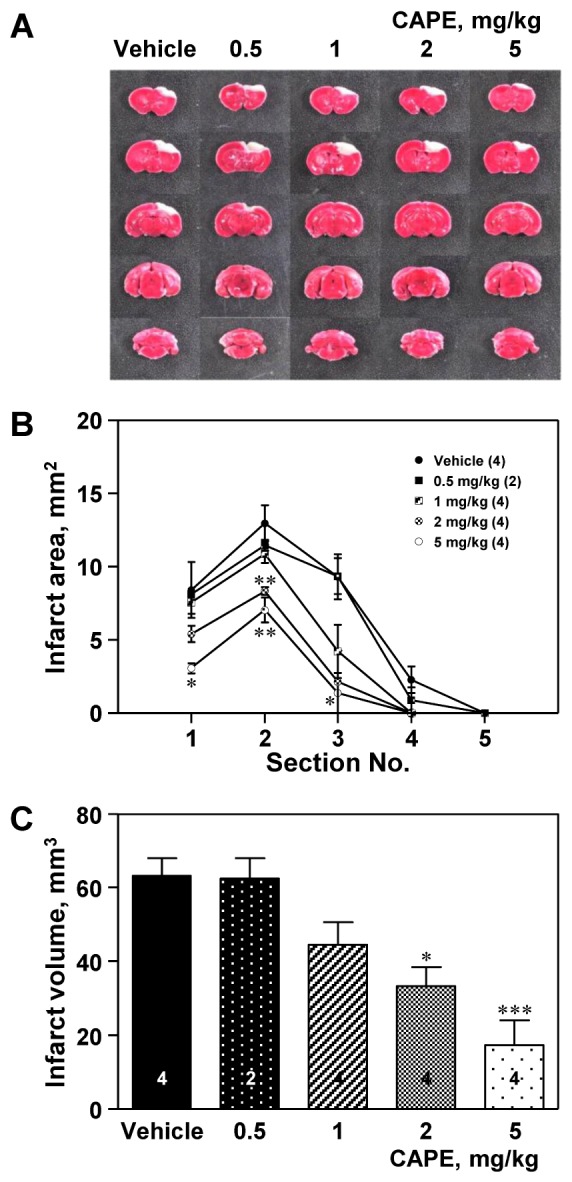
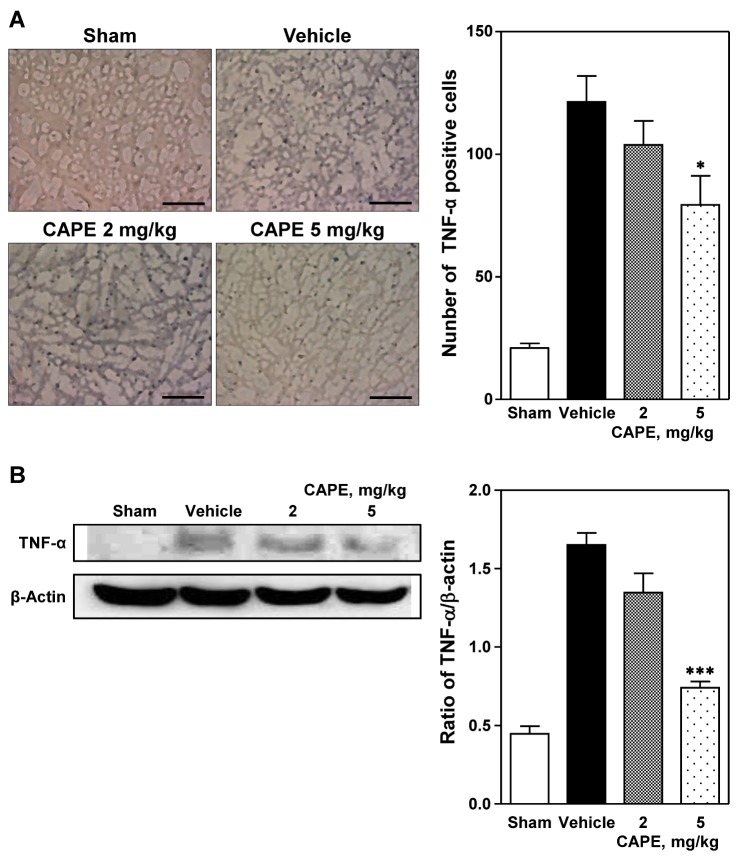
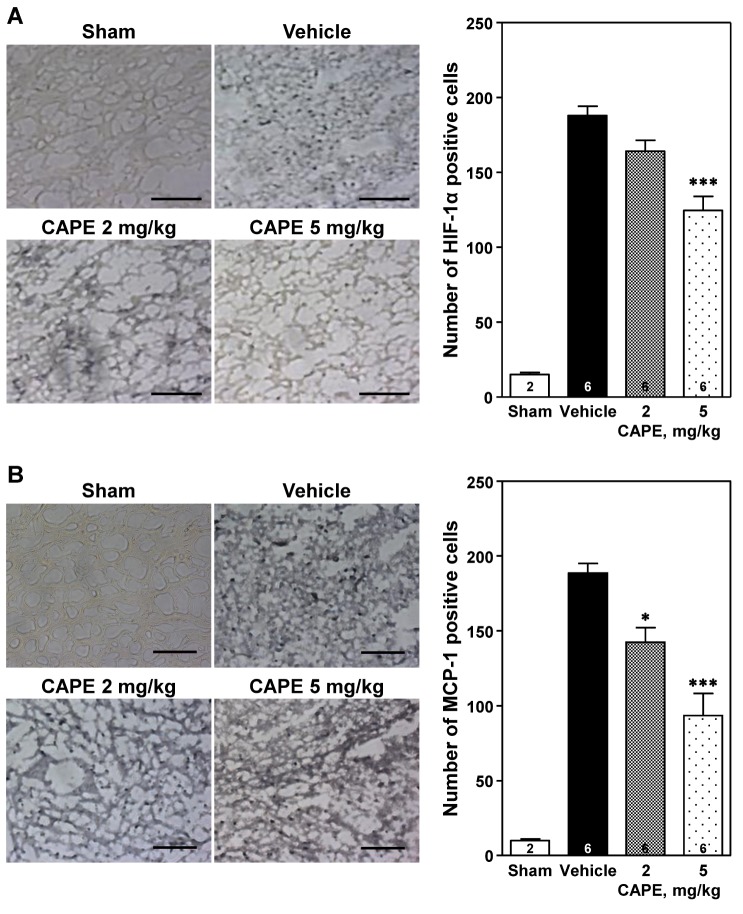
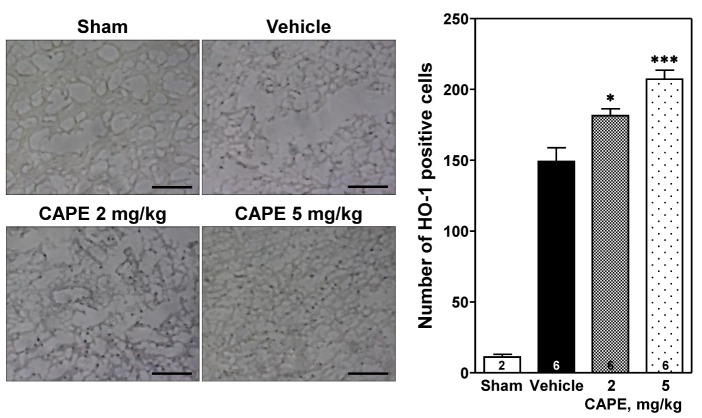




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download