Abstract
The sustained tonic currents (Itonic) generated by γ-aminobutyric acid A receptors (GABAARs) are implicated in diverse age-dependent brain functions. While various mechanisms regulating Itonic in the hippocampus are known, their combined role in Itonic regulation is not well understood in different age groups. In this study, we demonstrated that a developmental increase in GABA transporter (GAT) expression, combined with gradual decrease in GABAAR α5 subunit, resulted in various Itonic in the dentate gyrus granule cells (DGGCs) of preadolescent rats. Both GAT-1 and GAT-3 expression gradually increased at infantile (P6-8 and P13-15) and juvenile (P20-22 and P27-29) stages, with stabilization observed thereafter in adolescents (P34-36) and young adults (P41-43). Itonic facilitation of a selective GAT-1 blocker (NO-711) was significantly less at P6-8 than after P13-15. The facilitation of Itonic by SNAP-5114, a GAT-3 inhibitor, was negligible in the absence of exogenous GABA at all tested ages. In contrast, Itonic in the presence of a nonselective GAT blocker (nipecotic acid, NPA) gradually decreased with age during the preadolescent period, which was mimicked by Itonic changes in the presence of exogenous GABA. Itonic sensitivity to L-655,708, a GABAAR α5 subunit inverse agonist, gradually decreased during the preadolescent period in the presence of NPA or exogenous GABA. Finally, Western blot analysis showed that the expression of the GABAAR α5 subunit in the dentate gyrus gradually decreased with age. Collectively, our results suggested that the Itonic regulation of altered GATs is under the final tune of GABAAR α5 subunit activation in DGGCs at different ages.
The activation of synaptic and extrasynaptic γ-aminobutyric acid A receptors (GABAARs) generates phasic and tonic forms of inhibition (tonic GABAA current, Itonic), respectively [12], and has a profound influence on the hippocampal neural circuitry. Itonic is particularly interesting in the context of different ages because extrasynaptic GABAAR signaling is implicated in brain physiology and rage of pathophysiologies [34567]. Changes in extracellular GABA concentrations alter the relative contribution of specific GABAARs to Itonic as different receptor populations are recruited [8]. GABAARs containing the α5 subunit (α5-GABAARs) contribute to Itonic when the ambient GABA concentration increases, while at low ambient GABA concentrations the activation of δ subunit-containing receptors predominates [9]. In dentate gyrus granule cells (DGGCs), Itonic increases during initial postnatal maturation [1011], and further increases as adolescents mature into adulthood [12]. The age-dependent increase of Itonic in DGGCs may mirror the increased expression of δ-GABAARs in adults [13], which raises the question of whether and how a developmental change in α5-GABAARs alter Itonic at different ages.
GABA transporters (GATs) are members of a family of Na+-dependent neurotransmitter reuptake proteins. To date, four different GATs (GAT-1, GAT-2, GAT-3, and Betain/GABA transporter type 1) have been described in rat brain. Of these, GAT-1 is a primary neuronal GAT, while GAT-3 is commonly associated with glial cells [14]. Accordingly these two GAT subtypes are responsible for controlling extracellular GABA released from vesicular and non-vesicular sources, respectively [15]. In the hippocampus, GAT-1 predominantly determines the GABA concentration surrounding neurons, while GAT-3 activity is apparent with increased extracellular GABA concentration, especially when GAT-1 is blocked [16]. However, GAT-1 expression is low at early postnatal age, with GAT-3 expression dominating in that period [17]. Overall, it remains unknown whether and how the interaction between GAT-1 and GAT-3 modulates Itonic during postnatal brain maturation. In this study, we investigated the combined role of GAT-1 and GAT-3 in Itonic regulation in DGGCs at different ages; the results suggested that Itonic mirrored the changes in expression of extrasynaptic GABAARs activated by elevated extracellular GABA, according to the interrelationship between neuronal and glial GATs.
Male Sprague-Dawly rats purchased from Samtako Bio (Kyung Gi-Do, Korea) were housed under a 12/12 h light/dark schedule with free access to food and water until used. Animals were grouped by postnatal day (P), as follows: infantile (P7–9 and P14–16), juvenile (P21–23 and P28–30), adolescence (P36–37), and young adulthood (P42–44 and P49–51). Brains were rapidly extracted for electrophysiological recordings or Western blotting from animals anesthetized with ketamine and xylazine (80 mg/kg and 12 mg/kg, i.p., respectively). Animals in early infantile stage (P7–9) were euthanized by decapitation without anesthesia. All animal experimentation was conducted in compliance with the policies of Chungnam National University regarding the use and care of animals.
Patch-clamp recordings were obtained in acutely prepared coronal hippocampal slices from male rats, as described previously [618]. Briefly, slices were perfused with artificial cerebrospinal fluid (aCSF; in mM: NaCl 126, KCl 2.5, MgSO4 1, NaHCO3 26, NaH2PO4 1.25, glucose 20, ascorbic acid 0.4, CaCl2 1, pyruvic acid 2; pH 7.3~7.4; saturated with 95%O2–5%CO2) at a ~3 ml/min flow. Recordings were obtained at 32℃ using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). The series resistance was motored throughout the experiments. Neurons localized in the outer half of the granule cell layer were selected to minimize the effects of neurogenesis [19]. Patch pipettes were filled with a high Cl− containing solution (in mM): KCl 140, HEPES 10, Mg2+ATP 5, MgCl2 0.9, and EGTA 10. Current output was filtered at 2 kHz and digitized at 10 kHz (Digidata 1322A, pClamp 9 software, Axon Instruments). Itonic was defined as the difference between the holding current (Iholding) before and after application of the GABAA receptor blocker bicuculline (20 µM). Drugs were added to the perfusing aCSF solution at known concentrations. All drugs except NO-711 (Tocris, UK) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
All proteins from the dissected dentate gyrus (DGs) were lysed with 1× passive lysis buffer (Cell Signaling Technology, Danvers, MA, USA) and quantified using a Coomassie Protein assay kit (Bio-Rad, Hercules, CA, USA). Approximately 50 µg of protein was electrophoresed on a 10% sodium dodecyl sulfate polyacrylamide gel (SDS–PAGE) and transferred onto nitrocellulose membranes. The blots were blocked with 1× Tris buffered saline (TBS)-Tween 20 containing 3% bovine serum albumin (BSA) +2% heparan sulfate (HS) for 1 h at room temperature (5% TTBS; Gibco, USA). The blots were then incubated at 4℃ with primary antibodies against GABAAR α5 subunit, GAT-1, and GAT-3 (1:1,000; Millipore, USA) in 5% TTBS, respectively. The next day, the blots were incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000; Santa Cruz Biotechnology, USA). An enhanced chemiluminescence detection kit (ECL; Pierce, USA) was used to visualize antibody binding, and the intensity of the bands was measured using Image J software 1.42q (NIH, USA).
Electrophysiological recordings were obtained from a total of 114 DGGCs. In addition to blocking synaptic transmission, the GABAAR antagonist, bicuculline (BIC, 20 µM), induced a variable outward shift in the holding current (Iholding) in DGGCs.
Altered expression of neuronal and glial GATs could contribute to Itonic changes by altering the extracellular GABA concentration at different ages. To understand the age-dependent changes in GAT expression, we directly compared the expression of GAT-1 and GAT-3 in the DGs at different ages. Western blot analysis showed that the expression of GAT-1 and GAT-3 in the DGs gradually increased at P7, P14, and P21, and thereafter stabilized at P28, P35, and P42 (Fig. 1).
Changes in the expression and/or relative contribution of GAT-1 and/or GAT-3 may cause Itonic changes at different ages. To discriminate the functional role of neuronal and glial GATs in Itonic, we measured and compared the Itonic of DGGCs in the presence of selective GAT-1 and GAT-3 blockers at different ages.
Bath application of NO-711 (5 µM), a selective GAT-1 blocker [20], failed to induce consistent changes in Iholding at P6–8, while it induced a similar inward shift in Iholding (INO-711) at P13–15 (21.8±4.4 pA, n=7), P20–22 (20.8±6.1 pA, n=6), P27–29 (21.4±3.6 pA, n=5), and P34–36 (27.3±3.4 pA, n=7) (Fig. 2A). In a subset of experiments, we measured INO-711 in the presence of 1 µM GABA to confirm that GAT-1 activity facilitates Itonic at the early infantile age. GABA (1 µM)-induced Iholding shift were decreased with age (P6–8, 16.3±4.2 pA, P20–22, 7.6±1.7 and P34–36, 3.3±1.2 pA, n=5~6). Additional application of NO-711 (GABA+NO-711) caused a significant inward shift in Iholding even at P6–8, which was still much smaller than at P20–22 and P34–36 (p<0.01 in both cases; Fig. 2B).
To investigate the functional significance of changes in GAT-3 expression, a selective GAT-3 blocker, SNAP-5114, was used [14]. Because SNAP-5114 (100 and 300 µM) alone failed to elicit changes in Iholding, we sequentially applied NO-711 and NO-711+SNAP-5114. In the presence of NO-711, 300 µM SNAP-5114 induced a significant inward shift in Iholding (ISNAP-5114) at P6–8, P20–22, and P34–36. ISNAP-5114 was significantly smaller at P6–8 than at P20–22, and P34–36 (p<0.05 in both cases; Fig. 2C). Even in the presence of NO-711, 100 µM SNAP-5114 did not induce significant changes in Iholding at all tested age groups (Fig. 2C). Thus, in a subset of experiments, we assessed ISNAP-5114 in the presence of 1 µM GABA at different ages. GABA induced significant Iholding changes at P6–9 (22.6±3.5 pA, n=6), while it induced minimal changes in Iholding at P20–22 (1.3±1.0 pA, n=5) and P34–36 (1.9±0.9 pA, n=5). An additional 100 µM SNAP-5114 efficiently induced an inward shift in Iholding at P6–9, while it failed to cause significant changes in Iholding at P20–22 and P34–36 in the presence of 1 µM GABA (Fig. 2D). These results support the idea that GAT3 activity is apparent with an increased extracellular GABA concentration in the hippocampus [16].
To investigate the combined role of GAT-1 and GAT-3 in developmental Itonic changes, we measured and compared the Itonic of DGGCs in the presence of the nonselective GAT blocker, nipecotic acid (NPA), at different ages.
In contrast to selective GAT-1 or GAT-3 blockade, NPA (100 µM) induced a significant inward shift in the Iholding of DGGCs (INPA), blocked by BIC, at all tested age groups. Interestingly, INPA gradually decreased during the infantile and juvenile periods (P6–8, P13–15, P20–22, and P27–29), and stabilized in adolescence and young adulthood (P34–36 and P41–43) (Fig. 3A and B).
We hypothesize that the age-dependent INPA change may mirror the functional maturation of GABAARs rather than the upregulation of GAT expression in preadolescence, and that the large INPA may be due to a high ambient GABA level. Therefore, we directly compared INPA inhibition of L-655,708, an inverse agonist at the benzodiazepine binding site of α5-GABAARs [21]. The bath application of L-655,708 (5 µM) efficiently blocked INPA at all tested ages (p<0.01 in all cases; Fig. 3A). Interestingly, in agreement with gradual INPA attenuation with age, L-655,708-sensitive INPA also gradually decreased during preadolescence (Fig. 3B). As a result, the portion of L-655,708-sensitive total INPA did not differ by age.
To understand the functional changes in GABAARs according to age, we directly compared Itonic activated by exogenous GABA (5 µM) and their sensitivity to L-655,708 at different ages.
Itonic gradually decreases as infants mature into adolescence. The large INPA that characterized infantile periods (P6–8 and P13–15) gradually decreased at the juvenile (P20–22, and P27–29) and adolescent (P34–36) stages, and thereafter stabilized in young adults (P41–43) (Fig. 4A and B). The bath application of L-655,708 (5 µM) partially blocked Itonic in the presence of GABA at all tested ages (p<0.01 in all cases; Fig. 4A). In agreement with Itonic attenuation, L-655,708-sensitive Itonic gradually decreased in preadolescence (Fig. 4A and B). We observed a tendency for the portion of L-655,708-sensitive total Itonic to decrease with age, although this did not reach statistical significance.
In further experiments, we directly compared the expression of the GABAAR α5 subunit in DGs in the different age groups (Fig. 4C and D). Although we detected very low level of GABAARs α5 subunit immune reactivity at P7 and P14 in DGs, Western blot analysis showed that the expression of the GABAARs α5 subunit gradually decreased in the juvenile (P21 and P28) and adolescent (P35) periods, and thereafter stabilized in the young adults (P42). The degree of GABAARs α5 subunit expression was not further changed at P49 and P56 (data not shown).
The main findings of the present study were as follows: 1) agedependent increase in the INO-711 and ISNAP-5114 of DGGCs at infantile stages were partially consistent with the gradual increase in GAT-1 and GAT-3 expression in infantile and juvenile DGs; 2) the age-dependent decrease in INPA was compromised as GABAAR α5 subunit expression gradually decreased during preadolescence. Together, these findings suggest that, in addition to regulation of the ambient GABA concentration according to GAT activity, the change in age-dependent Itonic mirrored the altered expression and/or composition of extrasynaptic GABAARs during preadolescence.
GATs embedded in axon terminal membranes and/or astrocyte plasma membranes regulate ambient GABA levels. In many neural systems, GAT-1 antagonists alone result in a smaller Itonic versus that observed when both GAT-1 and GAT-3 are blocked [2223]. This was explained by the involvement of both GAT-1 and GAT-3 transporters in regulating extracellular GABA concentrations around neurons. Similarly, INPA was much larger than INO-711 in infantile and juvenile DGGCs in the present study. Interpretation of this difference is complicated because, as opposed to NO-711, NPA is a GAT substrate. In addition, NPA could result in heteroexchange for GABA by GATs [24]. However, given that the concentrations of NPA and NO-711 used in this study were, respectively, about five and ten times that of the IC50 used for GAT-1 blockade [14], a simple interpretation could be that NPA blocked GAT-3 more efficiently than NO-711 during the infantile stage. However, our results showed that the GAT-1 blocker alone, and the GAT-1 blocker with additional application of SNAP-5114 (100 µM), resulted in a similar average Itonic of 20 pA in DGGCs after the late infantile periods; these results contradict the idea that GAT-3 actively contributed to the Itonic of DGGCs. In the present study, SNAP-5114 facilitated Itonic at the concentration of 300 µM, which was near to the IC50 for GAT-1 blockade [14], further confounding the role of GAT-3 in the Itonic of DGGCs. Combined with the fact that both GAT-1 and GAT-3 expression increased until the late juvenile periods, these results appear to support a major role of GAT-1 in the GABA uptake regulating Itonic in the adult hippocampus [16]. In general, our results suggest that GAT-1 and GAT-3 play a primary and adjunctive role, respectively, in regulating Itonic of DGGCs in preadolescece.
As with other neurotransmitter transporters, GATs can also act in reverse mode and thus release GABA from cells. Indeed, GABA can be secreted from cells by the reversed transport direction of GATs, particularly during early postnatal stages [2526]. Thus, it is possible that GABA release via reversed GAT activity is integral in maintaining GABA levels that activate Itonic [27] in the infantile and early juvenile stages. However, our finding that GAT-1 and GAT-3 blockers always enhanced the Itonic of DGGCs suggest that the two transporters operate synergistically to promote GABA uptake, which was seen at all tested ages in our experiments.
Both the α5 and d subunit are key mediating components of the Itonic of DGGCs [28]. Alterations in GABA concentrations affect the relative contribution of specific GABAARs to Itonic as different receptor populations are recruited [8]. In the present study, BIC uncovered basal Itonic shown by the outward Iholding shift went over the initial level, especially when the inward shift in Iholding by exogenous GABA and GAT blockers was less than ~30 pA. α5–GABAARs contribute to Itonic when ambient GABA concentrations increase, while at low ambient GABA concentrations the activation of δ–GABAARs predominates [9]. In the present study, INPA was larger than INO-711 during preadolescence, which could be explained by an ambient GABA concentration sufficient to recruit additional α5–GABAARs in the presence of NPA, but not in NO-711. Our results showed that the portion of L-655,708-sensitive INPA ranged from ~27% to ~32%; this is consistent with previous findings showing that Itonic is mediated by α5–GABAARs and is responsible for ~29% of the total Itonic in DGGCs [28]. Thus, α5–GABAARs mediated INPA at all tested ages. Combined with the results whereby INPA and GABAAR α5 subunit expression gradually decreased with age, our results suggest that the age-dependent INPA decrease mirrors the functional decrease of α5–GABAARs rather than changes in GATs activity, during preadolescence.
However, there may be an as-yet undiscovered, non α5–containing GABAARs responsible for the large INPA observed during preadolescences. Indeed, in the present study, GABAAR α5 subunit immunoreactivity was not detectable in DGs at infantile stages. It is also notable that the small amplitude of INO-711 prevented us from comparing the sensitivity of INPA and that of INO-711 to L-655,708 in DGGCs. Regarding GABAARs activated in the presence of NPA, it is also noteworthy that NPA can directly activate GABAAR-like channels [29]. However, to the best of our knowledge, there is no information on the composition of GABAARs directly activated by NPA. However, it is still of interest that GABAAR α5 subunit expression gradually decreased with the postnatal development in various brain regions.
Overall, our results showed that GATs blockades elevated ambient GABA level sufficiently to harmonize with α5-GABAARs, resulting in an age-dependent Itonic decrease in preadolescent brains. Combined with the fact that GABAAR α5 subunit expression in the hippocampus is closely related to learning and memory in young adults [3031], selective pharmacological modulators, such as α5-GABAAR selective inverse agonists, may be effective in increasing cognitive performance in memory disorders [32]. Future studies are warranted to elucidate the pathophysiology of α5-GABAARs generating Itonic combined with GAT blockades in the developing brains of preadolescents.
Figures and Tables
 | Fig. 1Expression of GABA transporter-1 (GAT-1) and GABA transporter-3 (GAT-3) in the dentate gyrus at different ages.Western blot analysis showing neuronal GAT-1 (A) and glial GAT-3 expression (B) in the dentate gyrus at different postnatal days (P). The expression was normalized to the level detected at P7 and compared with the expression at each age group (n=5), shown by the bar graphs. **p<0.01 compared with P7 expression.
|
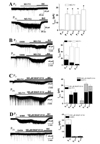 | Fig. 2Effects of NO-711 and SNAP-5114 on Itonic at different ages.(A) Representative current traces of dentate gyrus granule cell (DGGC) showing the effect of NO-711 (5 mM), a GAT-1 blocker. Note that the effects of NO-711, which were blocked by bicuculline (BIC), were smaller at P6-8 than in older age. Mean changes in the holding current (Iholding) after NO-711 application are summarized in the bar graph. (B) Representative current traces showing the effect of NO-711 in the presence of GABA (1 µM). Mean Iholding changes after the application of GABA and GABA+NO-711 are summarized in the bar graph. (C) Representative current traces showing the effect of SNAP-5114 (300 µM), a GAT-3 blocker, in the presence of NO-711 (5 µM). Note that the effects of SNAP-5114 were smaller at P6-8 than in older age. Mean changes in Iholding after by the sequential application of NO-711 and NO-711+SNAP-5114 (100 or 300 µM) are summarized. (D) Representative current traces showing the effect of SNAP-5114 (100 µM) in the presence of GABA (1 µM). Mean changes in Iholding after the application of GABA and GABA+SNAP-5114 (100 µM) are shown in the bar graph. Summarized data are shown as means±standard error of the mean (SEM) (n=5~6). *p<0.05, **p<0.01 compared with P6–8.
|
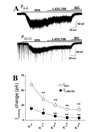 | Fig. 3Effects of nipecotic acid (NPA) on Itonic according to age.(A) Representative current traces before and during the sequential application of NPA (100 µM), a nonselective GAT blocker, and NPA+L-655,708, an inverse agonist of the GABAA receptor α5 subunit. Note that the effects of NPA, which were blocked by BIC, were larger at P6–8 than in older age. (B) Mean changes in the inward Iholding shift after NPA (INPA), and the outward Iholding shift following additional application of L-655,708 (IL,655–708), are summarized. Summarized data are shown as means±SEM (n=6~7). *p<0.05, **p<0.01 compared with P6–8.
|
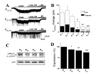 | Fig. 4Age-dependent decrease of GABAAR α5 subunit expression in preadolescent rats.(A) Representative current traces before and after the sequential application of GABA (5 µM) and GABA+L-655,708, an inverse agonist of the GABAA receptor α5 subunit. (B) Mean changes in the inward Iholding shift by GABA and outward Iholding shift following additional application of L-655,708 (IL,655–708) are summarized. Summarized data are shown as means±SEM (n=6~7). *p<0.05, **p<0.01 **, ***p<0.001 compared with P6–8. (C) Representative Western blot analysis showing GABAAR α5 subunit expression at different ages. (D) Summarized GABAAR α5 subunit expression at different ages. The protein expression was normalized to the level detected at P21. Summarized data are shown as means±SEM (n=4).*p<0.05, **p<0.01, ***p<0.001 compared with P21.
|
ACKNOWLEDGEMENTS
This work was supported by Chungnam National University and National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A02059430).
Notes
References
1. Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005; 6:215–229.
2. Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004; 27:262–269.
3. Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012; 73:23–34.
4. Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012; 22:552–558.
5. Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. The development of phasic and tonic inhibition in the rat visual cortex. Korean J Physiol Pharmacol. 2010; 14:399–405.
6. Pandit S, Jeong JA, Jo JY, Cho HS, Kim DW, Kim JM, Ryu PD, Lee SY, Kim HW, Jeon BH, Park JB. Dual mechanisms diminishing tonic GABAA inhibition of dentate gyrus granule cells in Noda epileptic rats. J Neurophysiol. 2013; 110:95–102.
7. Pandit S, Jo JY, Lee SU, Lee YJ, Lee SY, Ryu PD, Lee JU, Kim HW, Jeon BH, Park JB. Enhanced astroglial GABA uptake attenuates tonic GABAA inhibition of the presympathetic hypothalamic paraventricular nucleus neurons in heart failure. J Neurophysiol. 2015; 114:914–926.
8. Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003; 6:484–490.
9. Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005; 25:10016–10024.
10. Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 2010; 32:1300–1309.
11. Lee CY, Liou HH. GABAergic tonic inhibition is regulated by developmental age and epilepsy in the dentate gyrus. Neuroreport. 2013; 24:515–519.
12. Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007; 97:3806–3811.
13. Bright DP, Smart TG. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front Neural Circuits. 2013; 7:193.
14. Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996; 29:335–356.
15. Song I, Volynski K, Brenner T, Ushkaryov Y, Walker M, Semyanov A. Different transporter systems regulate extracellular GABA from vesicular and non-vesicular sources. Front Cell Neurosci. 2013; 7:23.
16. Kersanté F, Rowley SC, Pavlov I, Gutièrrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. A functional role for both-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol. 2013; 591:2429–2441.
17. Evans JE, Frostholm A, Rotter A. Embryonic and postnatal expression of four gamma-aminobutyric acid transporter mRNAs in the mouse brain and leptomeninges. J Comp Neurol. 1996; 376:431–446.
18. Pai YH, Lim CS, Park KA, Cho HS, Lee GS, Shin YS, Kim HW, Jeon BH, Yoon SH, Park JB. Facilitation of AMPA receptor-mediated steady-state current by extrasynaptic NMDA receptors in supraoptic magnocellular neurosecretory cells. Korean J Physiol Pharmacol. 2016; 20:425–432.
19. Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004; 429:184–187.
20. Suzdak PD, Frederiksen K, Andersen KE, Sørensen PO, Knutsen LJ, Nielsen EB. NNC-711, a novel potent and selective gamma-aminobutyric acid uptake inhibitor: pharmacological characterization. Eur J Pharmacol. 1992; 224:189–198.
21. Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996; 35:1331–1335.
22. Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol. 2010; 103:904–914.
23. Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005; 94:2073–2085.
24. Solís JM, Nicoll RA. Postsynaptic action of endogenous GABA released by nipecotic acid in the hippocampus. Neurosci Lett. 1992; 147:16–20.
25. Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002; 36:1051–1061.
26. Taylor J, Gordon-Weeks PR. Calcium-independent gamma-aminobutyric acid release from growth cones: role of gamma-aminobutyric acid transport. J Neurochem. 1991; 56:273–280.
27. Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003; 90:1363–1374.
28. Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008; 28:1421–1426.
29. Barrett-Jolley R. Nipecotic acid directly activates GABA(A)-like ion channels. Br J Pharmacol. 2001; 133:673–678.
30. Gill KM, Grace AA. The role of α5 GABAA receptor agonists in the treatment of cognitive deficits in schizophrenia. Curr Pharm Des. 2014; 20:5069–5076.
31. Prut L, Prenosil G, Willadt S, Vogt K, Fritschy JM, Crestani F. A reduction in hippocampal GABAA receptor alpha5 subunits disrupts the memory for location of objects in mice. Genes Brain Behav. 2010; 9:478–488.
32. Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol Ther. 2010; 125:11–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download