Abstract
Orthostatic hypotension (OH) is associated with symptoms including headache, dizziness, and syncope. The incidence of OH increases with age. Attenuation of the vestibulosympathetic reflex (VSR) is also associated with an increased incidence of OH. In order to understand the pathophysiology of OH, we investigated the physiological characteristics of the VSR in the disorder. We applied sodium nitroprusside (SNP) to conscious rats with sinoaortic denervation in order to induce hypotension. Expression of pERK in the intermediolateral cell column (IMC) of the T4~7 thoracic spinal regions, blood epinephrine levels, and blood pressure were evaluated following the administration of glutamate and/or SNP. SNP-induced hypotension led to increased pERK expression in the medial vestibular nucleus (MVN), rostral ventrolateral medullary nucleus (RVLM) and the IMC, as well as increased blood epinephrine levels. We co-administered either a glutamate receptor agonist or a glutamate receptor antagonist to the MVN or the RVLM. The administration of the glutamate receptor agonists, AMPA or NMDA, to the MVN or RVLM led to elevated blood pressure, increased pERK expression in the IMC, and increased blood epinephrine levels. Administration of the glutamate receptor antagonists, CNQX or MK801, to the MVN or RVLM attenuated the increased pERK expression and blood epinephrine levels caused by SNP-induced hypotension. These results suggest that two components of the pathway which maintains blood pressure are involved in the VSR induced by SNP. These are the neurogenic control of blood pressure via the RVLM and the humoral control of blood pressure via epinephrine release from the adrenal medulla.
An instantaneous standing motion leads to a reduction of cerebral blood flow. This in turn induces a reflex that causes an increase in the sympathetic activity of the vasoconstrictor muscle, in order to prevent a further decline in cerebral blood flow. However, if the sympathetic activity and vascular resistance cannot be increased, the retention of peripheral venous blood and the changes in the distribution of central blood will result in orthostatic hypotension (OH). OH is defined as a 20 mmHg decrease in systolic blood pressure or a 10 mmHg decrease in diastolic blood pressure, occurring within three minutes of a postural change. It is accompanied by changes in blood supply to the central nervous system, resulting in headache, dizziness, and syncope [1]. Following a sudden rise from a seated position, 500~1,000 ml of blood is stored in the lower limb and internal circulation, and the amount of blood circulating to the heart is reduced. This results in a decrease in cardiac output and blood pressure. This change in hemodynamics leads to the baroreceptor reflex, which activates the sympathetic nervous system to cause an elevation in blood pressure [23]. In addition, a postural change leads to excitation of the vestibular receptors, inducing the vestibulosympathetic reflex (VSR). This is also involved in the elevation of blood pressure. The interaction of the baroreceptor reflex and the VSR is related to compensatory control of blood pressure. It can lead to the observed decrease in blood pressure following a postural change. If these blood pressure control reflexes do not function normally, OH will occur [45].
OH is a clinically important condition in the elderly as it is associated with an increased mortality rate in older people. It is caused by the hypofunction of the baroreceptor reflex, a decrease in cardiac compliance, or hypofunction of the VSR. A reduced VSR is commonly seen in the elderly due to functional and anatomical changes in the central and peripheral vestibular system [6]. Changes in arterial blood pressure evoke the excitation of baroreceptors in the carotid sinus and aortic arch, leading to the baroreceptor reflex. The signals from the baroreceptors are conveyed by branches of the glossopharyngeal and vagus nerves to the rostral ventrolateral medulla (RVLM), resulting in restoration of blood pressure [2].
The vestibular system located in the inner ear controls posture and movement. It also plays a role in autonomic function, through the sympathetic nervous system [78]. Natural or electrical stimulation of the vestibular receptors excites peripheral sympathetic nerves via the medial and inferior vestibular nuclei [910]. Electrical stimulation of the vestibular receptors or vestibular nuclei causes an elevation in blood pressure [11]. The VSR is known to be involved in blood pressure regulation. Signals from the vestibular receptors are transmitted to the vestibular nuclei and then to the RVLM via the solitary tract nucleus and caudal ventrolateral medulla, or to the RVLM directly from the vestibular nuclei [9121314]. Therefore, the VSR convergences with the baroreceptor reflex in the solitary tract nucleus and RVLM to regulate blood pressure [15]. The VSR excites sympathetic nerves via the vestibular nuclei and RVLM [9151617]. However, there is little information regarding the connection between the vestibular receptors and the sympathetic neurons of the spinal cord or the adrenal medulla. Elucidating the pathway from the vestibular receptors to the sympathetic neurons of the spinal cord and establishing the changes in the sympathetic neurotransmitters following a stimulation of the vestibular receptors, will provide a clearer explanation of the VSR and pathophysiology of orthostatic hypotension.
To establish the pathway of the VSR which is involved in blood pressure changes, it would be reasonable to stimulate the vestibular receptors by inducing hypotension. Acute hypotension in experimental animals leads to a decreased blood flow to the inner ear, which results in excitatory toxicity of the vestibular hair cells [818]. Excitatory signals from the vestibular receptors are transmitted to the vestibular nuclei and may lead to dizziness. Hypotension increases the electrical activity and immunoprotein expression of the vestibular nuclei. These results are not observed following a bilateral labyrinthectomy [819]. Unlike the baroreceptors, the vestibular receptors are sensitive to a decrease in blood pressure, but not to the increase of blood pressure. This means that the VSR is primarily involved in the occurrence of OH [15].
Glutamate is an important neurotransmitter involved in the VSR. Hypotension leads to an increased release of glutamate in the vestibular nuclei. Excitatory signals due to hypotension are transmitted through glutamate NMDA and AMPA receptors [18]. It has also been reported that glutamate acts as an important excitatory neurotransmitter in the RVLM [20]. Therefore, the measurement of glutamate neurotransmitters may systematically pursue the VSR pathway.
Immunohistochemical methods are useful for spatio-temporal integration studies of neural networks in the central nervous system [21]. Immunohistochemical methods can be used to observe the activities of various regions in the central nervous system simultaneously. Typically, c-Fos and pERK are used in immunohistochemical analysis of spatio-temporal integration studies. c-Fos is observed only in the nucleus. Following an external stimuli, c-Fos protein expression typically occurs within 30~60 minutes [22]. As pERK is expressed not only in the nucleus, but also in the cytoplasm and dendrites, following a short stimulation of a few seconds to several minutes, pERK protein is expressed within 5~10 minutes. Due to this property, pERK is more widely used than c-Fos to measure the spatio-temporal characteristics of neural networks [2123].
This study examined the neural pathways behind the neurogenic and humoral control of the VSR, in relation to OH. The experiment was split into three components. 1) We induced hypotension in rats by applying SNP. In order to observe the VSR pathway involved in the control of blood pressure, pERK expression was measured in the medial vestibular nuclei (MVN), RVLM, and the intermediolateral cell column (IMC) of T4~7 thoracic spinal regions following SNP application. 2) To observe the neurogenic control of the VSR, pERK was measured in the IMC, following the administration of glutamate to the MVN and RVLM. 3) To observe the humoral control of the VSR, blood epinephrine level was measured following the administration of glutamate to the MVN and RVLM.
In all of the experiments, sinoaortic denervated (SAD) animals were used to discard the role of the baroreceptor during hypotension. Non-anesthetized rats were used to exclude the effect of anesthetics on the expression of pERK [24].
For the experiment, mature Sprague-Dawley male rats, (Samtaco, Osan) weighing 250 g, were used. The animals were divided into a control group, with normal blood pressure, and an experimental group, with SNP-induced hypotension. The experimental group was split into a group where the MVN was tested and a group where the RVLM was investigated. Each group was subdivided again and microinjected with either ACSF, NMDA, AMPA, ACSF+SNP, MK801+SNP or CNQX+SNP, for the analysis of the changes in pERK and epinephrine (n=6 in each subgroup). Prior to the experiment, the afferent nerves from the baroreceptors were removed from all animals, in order to exclude baroreceptor reflex. To measure pERK expression and the blood concentration of epinephrine, no anesthesia was used. The experimental design and method complied with the regulations of Wonkwang University Experimental Animal Ethics Committee, and efforts were made to minimize the pain of the experimental animals and to minimize the number of sacrificed animals.
The carotid sinus nerve and the aortic arch nerve were removed under anesthesia with isoflurane (Ilsung Pharmaceutical Co., Seoul, Korea), to exclude the baroreceptor reflex. The skin was incised along the midline of the ventral neck. The muscles located in front of the bronchus and carotid artery branch were exposed, and the sternohyoid muscle was excised. The vagus nerve was separated from the carotid artery. In order to remove the carotid sinus nerve, the external, internal, and common carotid arteries were exposed. Following this, all of the connective tissues were dissected at the bifurcation of the external and internal carotid arteries, and 10% phenolethanol was applied. Care was taken to not damage the vagus nerve with the phenolethanol solution. The aortic arch nerve was severed at the proximal junction with the vagus nerve and removed [25]. All of the procedures were performed under a surgical microscope. The removal of the baroreceptor reflex was confirmed by the loss of reflex bradycardia induced by phenylephrine (3 µg/kg, i.v.) and the loss of reflex tachycardia induced by SNP (5 µg/kg, i.v.). After surgery, all of the animals maintained normal respiration, with no significant changes in the respiratory cycle. SAD was performed in all animals 24 h before the experiment.
The anesthetized animals were fixed in a stereotaxic instrument (Narishige, Tokyo, Japan), and the scalp was incised at the midline of the skull. A hole was made in the skull based on a stereotaxic atlas [26]. A microtrocar was inserted into the left MVN and RVLM and fixed with dental cement. The microtrocar for the MVN was placed 11.6 mm posterior to the bregma, 1.5 mm left from the midline, and 6.4 mm depth from the dura mater. The microtrocar for the RVLM was located 12.7 mm posterior to the bregma, 2.4 mm left of the midline, and 7.3 mm depth from the dura mater [26]. 10 µl of either ACSF, NMDA, AMPA, MK801, or CNQX (all 1 mM) was injected into the MVN or RVLM using a microinjector (Harvard Apparatus, Holliston, MA, USA). All of the animals were dosed while maintaining consciousness. Hypotension was induced by an intravenous injection of SNP, 10 min after drug injection. After the end of the experiment, the MVN or RVLM were histologically confirmed by a stereotaxic atlas [26].
A heparinized polyethylene tube was inserted into the femoral artery under isoflurane anesthesia, in order to measure blood pressure with a physiological recorder (Grass model 7400, Grass Technologies, Warwick, RI, USA). A second tube was inserted into the femoral vein for the injection of SNP. Both tubes were guided subcutaneously to the skull and fixed to the skull using screws. The tubes were connected to a cybernation metabolism cage, to allow the animal to move freely during the experiment. SNP was injected through the femoral vein at a dose of 15 µg/kg in order to reduce the blood pressure by 30~40 mmHg. The control group was injected with an identical quantity of physiological saline.
After 10 min of SNP administration, sodium pentobarbital (100 mg/kg) was intraperitoneally injected and the animals were sacrificed. The heart was perfused with phosphate buffered saline (PBS) solution (pH 7.4). The brain and spinal cord were removed after perfusion with 4% paraformaldehyde. The brain and spinal cord were fixed in 4% paraformaldehyde at room temperature for 3 h, then left in 30% sucrose for greater than two days. Tissue slices, with a thickness of 20 µm, were prepared using a Cryostat (Leica; Nubloch, Germany) and placed onto a slide glass. They were mixed in a 3% H2O2 solution for 30 min, washed three times with PBS (pH 7.4), shaken in a 0.3% Triton-X 100 for 30 min and then washed three more times with PBS. The samples were treated with blocking agent (normal goat serum) for 30 min at room temperature. Primary anti-rabbit polyclonal anti-ERK1/2 antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA) was then applied for overnight. The samples were then shaken at room temperature for 2 h and washed with PBS. The samples were then treated with biotinylated goat anti-rabbit (1:200; Vector Labs; Burlingame, CA, USA) at room temperature for 40 min, washed with PBS, and then treated with streptavidin peroxidase (ABC kit; Vector Labs; Burlingame, CA, USA) for 20 min. After washing with PBS, the samples were developed in chromogen 0.05% diaminobenzidine. Finally, the samples were washed for 1 h in distilled water. Dark brown pERK1/2 positive cells were observed by light microscopy in the MVN, RVLM, and IMC of the T4~7 thoracic spinal regions. The number of pERK1/2 positive cells was measured using an image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA).
The animals were sacrificed in the same manner as above. The T4~T7 spinal cord was rapidly cut into cross-sections, in cold artificial cerebrospinal fluid. The IMC was selectively removed using a tissue puncture (1.8 mm in ID: Fine Science Tools, USA). For protein extraction, the tissues were placed into a 5-fold tissue volume of tissue degradation buffer (25 mM Tris, 1 mM EGTA, 1 mM DTT, 0.1% Triton X-100) and ground. After centrifugation (12,000 g, 4℃) for 15 min, the supernatant was collected and quantified using a NanoDrop. The supernatant was electrophoresed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). A 1/5 volume of sample buffer (60 mM Tris, 2% SDS, 25% glycerol, 0.1% bromophenol blue, 14.4 mM 2-mercaptoethanol) was added to the quantified protein and the mixture was shaken with boiling water. It was then electrophoresed on 10% polyacrylamide gel. Electrophoresis was carried out using a mini gel electrophoresis apparatus (Bio-Rad, USA) (100 V) for 2 h. After electrophoresis, the proteins were transferred to a 0.45 µm polyvinylidene difluoride (PVDF) membrane (Millipore, USA) at 45 V for 2 h, using a protein transfer system (Bio-Rad, USA). The PVDF membrane was washed with Tris buffered saline (TBS, pH 7.4) and incubated in 3% bovine serum albumin for 30 min. The primary antibody, anti-pERK1/2 was left on the samples overnight at 4℃. They were then washed three times with 0.05% Tween 20-TBS (TBS-T) solution. The secondary antibody, anti-rabbit IgG conjugated HRP (Santa Cruz, USA) was diluted to 1:1,000 and left on the samples for 1 h. The samples were washed three times with TBS-T, then reacted in an enhanced chemiluminescent (ECL) solution. Finally, they were photographed with Fluorchem E Digital Darkroom System (Select Science, UK) in order to identify specific proteins.
Immediately after the injection of SNP or glutamate, blood was collected from the femoral artery and stored in a polyethylene tube containing the anticoagulant ethylene diamine tetra acetic acid. It was then centrifuged at 4℃ for 10 min (1,000 × g). After centrifugation, the supernatant was rapidly frozen at −25℃. The concentration of epinephrine was measured using an enzyme-linked immunosorbent assay (ELISA) (TSZ Scientific LLC, Lexington, MA., USA) with a mutual diversity of less than 10%.
In order to investigate the effect of SNP on the reduction of blood pressure, blood pressure was measured in a stable state before the administration of SNP. Blood pressure was taken both of animals in which the baroreceptors and vestibular receptors were both intact, and animals where the baroreceptors had been removed (SAD). The mean arterial blood pressure was 105.1±8.5 mmHg in intact animals and 99.3±9.7 mmHg in SAD animals. Intravenous injection of SNP reduced blood pressure within 1 min, and the decrease in blood pressure persisted for 2 min. Following SNP injection, the mean arterial blood pressure of the animals with both receptors intact was 72.5±7.9 and in SAD animals was 70.9±8.6 mmHg. Both results were significantly decreased compared to the results prior to SNP injection (p<0.01). To investigate the effect of the vestibular nuclei on blood pressure control, blood pressure was measured after the administration of glutamate to the MVN. Before drug administration, in the stable state, mean arterial blood pressure was 97.7±9.2 mmHg. In the control group, ACSF was administered to the MVN and no changes in blood pressure were observed. However, administration of the glutamate agonist NMDA or AMPA to the MVN increased blood pressure by 20~30 mmHg (Fig. 1).
To investigate the effect of SNP-induced hypotension on the vestibular nucleus, medulla, and sympathetic neurons of the spinal cord, pERK expression was measured in the MVN, RVLM, and IMC of the T4~7 spinal regions. The animals with intact baroreceptors and vestibular receptors and the animals with SAD presented with between two and three pERK-positive neurons in the stable state, therefore the removal of the baroreceptors did not affect pERK expression. SNP-induced hypotension led to a significant increase in pERK expression in animals with SAD. The expression was highest 10 min after SNP administration, and gradually decreased thereafter. Expression disappeared after 60 min. After 10 min of SNP administration, pERK-positive neurons were counted as: 65.7±16.1 in the MVN, 55.9±4.1 in the RVLM, and 20.6±1.2 in the IMC (Fig. 2).
To confirm the functional connectivity between the vestibular nucleus and the spinal sympathetic neurons, pERK expression in the IMC of T4~7 spinal regions was measured after the administration of glutamate to the MVN. ACSF was administered to the MVN of SAD animals as a control. In the controls, 1.5±0.4 pERK-positive neurons were observed in the IMC. However, the administration of the glutamate agonist AMPA or NMDA to the MVN, resulted in a marked increase in the number of pERK-positive neurons, compared with the control group. Following the administration of AMPA, 21.5±0.9 pERK-positive neurons were observed and following the application of NMDA, 20.0±1.1 neurons were counted (p<0.01). In order to investigate the effect of a glutamate antagonist, pERK expression was measured in the IMC after the administration of CNQX or MK801 to the MVN, in SNP-induced hypotensive animals. In the SNP-treated animals, following administration of ACSF to the MVN as a control, 19.5±0.6 pERK-positive neurons were counted in the IMC. Following pretreatment with CNQX 1.7±0.3 pERK-positive neurons were observed and following MK801 application 3.0±0.4 pERK-positive neurons were seen, which was significantly decreased compared to the ACSF control (p<0.01) (Fig. 3).
To confirm the functional connectivity between the RVLM and the spinal sympathetic neurons, pERK expression in the IMC of T4~7 spinal regions was measured after the administration of glutamate to the RVLM. As a control, ACSF was administered to the RVLM in the animals with SAD. In these animals, 2.8±0.7 pERK-positive neurons were observed in the IMC. The administration of the glutamate agonist AMPA or NMDA to the RVLM resulted in a marked increase compared to the control group. Following AMPA administration 22.8±0.9 positive neurons were observed and following NMDA application, 21.3±1.2 neurons were counted (p<0.01). In order to investigate the effect of a glutamate antagonist, pERK expression was measured in the IMC following the administration of CNQX or MK801 to the RVLM in SNP-induced hypotensive animals. In the SNP-treated animals, following pretreatment with ACSF to the RVLM 24.7±1.4 pERK-positive neurons were observed in the IMC. Following pretreatment with CNQX 4.3±0.9 positive neurons were counted and following MK-801 administration, 6.3±1.5 pERK-positive neurons were seen, which was a marked decrease compared to the control group (p<0.01) (Fig. 4).
To confirm the functional connectivity between the MVN and the IMC, following the administration of glutamate to the MVN, the level of pERK expression in the IMC of the T4~7 spinal regions was measured using a Western blot. The relative expression level of pERK in the IMC was 2.9±0.3 after administration of ACSF to the MVN in the animals with SAD (as a control). After the administration of AMPA or NMDA to the MVN, the relative expression level was 5.4±0.4 or 4.3±0.3 respectively, which in both cases was significantly increased compared to the control group (p<0.05). In order to investigate the effect of glutamate antagonist, the relative expression level of pERK was measured in the IMC after administration of CNQX or MK801 to the MVN in SNP-induced hypotensive animals. In the MVN of SNP-treated animals which were pretreated with ACSF as a control, a relative expression level of 4.5±0.5 pERK levels was observed in the IMC. Pretreatment with CNQX or MK801 to the MVN resulted in 2.9±0.3 or 3.3±0.2 of relative expression level, respectively, which showed significant decrease compared with ACSF pretreatment (p<0.05). The expression of pERK observed in Western blotting was consistent with the pattern of pERK-positive neurons measured by immunohistochemistry (Fig. 5).
In order to investigate the effect of the administration of glutamate on blood epinephrine levels, epinephrine was measured after the administration of glutamate to the MVN or RVLM in animals with SAD. The blood epinephrine level was 76.9±1.2 pg/ml following the administration of ACSF to the MVN as a control. After the administration of AMPA or NMDA to the MVN, blood epinephrine levels were 96.8±3.5 or 89.2±2.2 pg/ml, respectively, a significant increase compared to the control group (p<0.05). In order to investigate the effect of a glutamate antagonist, blood epinephrine levels were measured following the administration of CNQX or MK801 to the MVN in SNP-induced hypotensive animals. In the SNP-treated animals, ACSF was applied to the MVN as a control. Following this, 92.3±4.8 pg/ml epinephrine were measured in the blood. This was significantly increased compared to animals with normal blood pressure (p<0.01). The application of CNQX or MK801 to the MVN resulted in 73.5±1.8 or 78.9±3.3 pg/ml of blood epinephrine, respectively. These results were significantly decreased compared to the control group (p<0.05).
ACSF was applied to the RVLM as a control. Following this, the blood epinephrine level was 75.1±1.2 pg/ml. After the administration of AMPA or NMDA, blood epinephrine levels were 90.8±1.5 or 82.9±1.7 pg/ml, respectively. These results were significantly increased compared to the control group (p<0.05). In the SNP-treated animals, blood epinephrine was found to be 91.2±1.9 pg/ml after the pretreatment with ACSF to the RVLM as a control. This was significantly increased compared to animals with normal blood pressure (p<0.01). Following the application of CNQX or MK801 to the RVLM, epinephrine levels were 79.4±1.5 or 80.2±1.3 pg/ml, respectively. These results were significantly decreased compared to the control group (p<0.05) (Fig. 6).
The vestibular system of the inner ear uses vestibuloocular and vestibulospinal reflexes to assist in the reflex control of body posture. It plays a role in the autonomic regulation of blood pressure and respiration, as postural changes occur. Postural changes stimulate peripheral vestibular receptors which convert mechanical stimuli into electrical signals. These signals are transmitted to the vestibular nucleus in the brainstem [7]. The vestibular nucleus transmits the efferent signal to the oculomotor nucleus and the alpha motor neuron of the spinal cord. This induces a reflex ocular movement and skeletal muscle contraction. The vestibular nucleus also transmits an efferent signal to the RVLM of the sympathetic nervous system. This causes the VSR, which helps to regulate autonomic nervous system function. The VSR is a reflex pathway in which afferent signals from the semicircular canals and otolith organs are transmitted to the solitary tract nucleus, caudal ventrolateral medulla and RVLM via the inferior portion of the MVN and the inferior vestibular nuclei [2728]. Although vestibuloocular and vestibulospinal reflexes have been studied for a long time [2930], investigation of the VSR is a relatively new field of interest.
Several studies have reported changes in sympathetic nervous activity and blood pressure following postural changes [8313233]. Doba and Reis [34] observed that when the head was elevated, a significant decrease in blood pressure occurred following the removal of the bilateral peripheral vestibular receptors in cats. This suggested that the vestibular receptors may be associated with the induction of OH. The increased incidence of OH in elderly patients is due to hypofunction of the vestibular system which leads to a weakened VSR [35].
In this study, we used an animal model of hypotension to observe the functional pathway of VSR in OH. SNP reduces blood pressure by leading to the expansion of blood vessels by the release of nitric oxide [36]. In this study, SNP decreased the blood pressure by 30~40 mmHg. Blood pressure reduction results in a decreased blood flow to the vestibular receptors, leading to excitatory toxicity in the vestibular hair cells. This excitatory toxicity results in the excitation of the vestibular receptors [18]. Therefore, the induction of hypotension by SNP administration increases the electrical activity of vestibular neurons and increases expression of early genes such as c-Fos and pERK. This electrical activity and early gene expression stimulated by the vestibular neurons is lost following the bilateral removal of the vestibular receptors. Therefore, SNP-induced hypotensive animal models can be used to study OH [81937].
Hypotension has been reported to increase glutamate release in the vestibular nuclei. Excitatory signals are then transmitted by the glutamate NMDA receptor and the AMPA receptor in the vestibular nuclei [1838]. The administration of the glutamate agonists AMPA or NMDA to the vestibular nucleus leads to an increase in blood pressure. This suggests that excitation of the vestibular neurons increases the blood pressure through the sympathetic nervous system. In turn, this indicates that there are functional connections between the vestibular nuclei and the sympathetic nervous system. These results are consistent with the previous studies in which it was found that stimulation of the vestibular system leads to increased activity in the peripheral sympathetic nerves [93940]. This suggests that the decrease in blood pressure caused by SNP leads to an increased glutamate release in the vestibular nuclei and eventually an increase in sympathetic nerve activity.
In order to assess the neural pathway which connects the vestibular nuclei to the induction of elevated blood pressure, pERK expression was observed. Following stimulation of the neurons, the early gene pERK is rapidly expressed in the nucleus, cytoplasm, and dendrites. We found that excitation of the vestibular receptors caused by hypotension, increased pERK expression in the MVN. This is evidence that the excitatory signals induced by hypotension are transmitted from the vestibular receptors to the vestibular nuclei, in agreement with a study which found that expression of c-Fos is increased in the MVN after the occurrence of hypotension [37]. In this study, we excluded the effect of baroreceptor reflex on blood pressure changes by performing SAD. In stable state, SAD animals showed only low levels of pERK expression in the MVN. These levels were not significantly different to those in normal, healthy animals. This indicated that SAD does not affect the vestibular nuclei. SNP-induced hypotension leads to increased pERK expression in the RVLM, IMC and the MVN. This indirectly indicates that the vestibulosympathetic systems are involved in control of blood pressure. These results are consistent with a report in which hydralazine induced hypotension was found to lead to an increased expression of pERK in the RVLM, solitary tract nucleus, parabrachial nucleus, and locus coeruleus [21].
Following the administration of a glutamate agonist to the MVN, expression of pERK was significantly increased in the IMC. The increased expression of pERK due to hypotension was lost following administration of glutamate antagonists to the MVN. This indicated that the MVN and IMC are functionally connected. As sympathetic neurons are located in the IMC, this result suggests that there is a functional connectivity between the MVN and sympathetic nervous system.
The RVLM is the center of the sympathetic nervous system and plays an important role in the regulation of blood pressure through the baroreceptor reflex. Afferent signals from the baroreceptors reach the caudal ventrolateral medulla via the solitary tract nucleus. The caudal ventrolateral medulla sends inhibitory signals to the RVLM, in which glutamate acts as the main neurotransmitter [2]. In addition, the RVLM has been shown to directly receive input signals from the vestibular nuclei [28]. In the present study, as the baroreceptors were removed, the RVLM was predominantly stimulated by afferent signals from the vestibular receptors. Expression of pERK in the IMC of T4~7 spinal regions was measured after the administration of glutamate to the RVLM. As SNP-induced hypotension led to increased pERK expression in the MVN, RVLM, and the spinal cord, this allowed us to identify the neural connections between the RVLM and the IMC. Application of a glutamate agonist led to increased pERK expression in the IMC. The application of a glutamate antagonist led to the loss of the observed increase in pERK expression which occurred in control animals following the induction of hypotension. This was consistent with our findings which investigated the application of glutamate to the MVN. These results suggest that during SNP-induced hypotension, the RVLM and the IMC are functionally linked. The pattern of pERK expression in the MVN, RVLM, and IMC following SNP-induced hypotension, was similar to our previous study in which the expression of c-Fos protein was measured [17].
In this study, pERK was measured by performing immunohistochemistry on the IMC of T4~7 cross-sections. This method can provide very fragmentary information in T4~7. In order to compensate for this, the T4~7 spinal regions were selected as they correspond to the location of the IMC, and the amount of pERK protein was measured by Western blot [41]. Following the administration of a glutamate agonist to the MVN, the amount of pERK protein was shown to increase. SNP-induced hypotension leads to increased levels of pERK expression. When a glutamate antagonist was applied to hypotensive animals, this increase in pERK levels did not occur. Western blot results were consistent with the immunohistochemical data, indicating the reliability of the immunohistochemical method.
The sympathetic nerves originate from the IMC of T1 to L2 and project preganglionic fibers to the IMC from the RVLM. The sympathetic nerves responsible for the innervation of the adrenal gland reside in the IMC at levels T4~7. In the IMC, pERK expression increases following SNP-induced hypotension or glutamate administration to the MVN or RVLM. The sympathetic nerves are stimulated following the excitation of the vestibular receptors by hypotension. These results are consistent with the neurogenic control of the VSR, whereby decreased blood pressure leads to excitation of the vestibular receptors which then transmit signals through the vestibular nucleus, RVLM, and IMC of the thoracic cord [174243].
The adrenal gland is composed of chromaffin cells which secrete catecholamines and dopamine. Catecholamines consist of epinephrine (85%) and norepinephrine (15%) and are involved in the control of blood pressure. Both SNP-induced hypotension and the application of glutamate agonists to the MVN or RVLM, led to significantly increased blood epinephrine levels. However, the increased blood levels of epinephrine following SNP-induced hypotension did not occur after pretreatment with a glutamate antagonist. This response was consistent with the pERK expression patterns in the T4~7 IMC, suggesting that the increase in blood epinephrine levels was associated with increased pERK expression in the T4~7 IMC. Therefore, in the humoral control of the VSR, hypotension led to the excitation of the vestibular receptors. In turn, these caused spinal sympathetic nerve stimulation via the vestibular nuclei and the RVLM. The excitatory signals from the spinal sympathetic nerves were transmitted to the adrenal gland from which epinephrine was released, leading to an increase in blood pressure.
Both SNP-induced hypotension and the administration of glutamate led to increased expression of pERK in the IMC and increased blood epinephrine levels. When there is a decrease in blood pressure, the vestibular receptors generate excitatory signals which are transmitted to the RVLM through the vestibular nuclei. There is then a compensatory increase in blood pressure by the direct innervation of sympathetic nerves to the heart and blood vessels. This pathway represents the neurogenic control of the VSR. The dual action between the neurogenic and humoral control of the VSR is a characteristic of vestibular function that differs from the postural control reflex.
Baroreceptors, vestibular receptors, muscular receptors, and mechanical receptors of the skin are involved in the reflex control of blood pressure [4445]. Afferent signals from the baroreceptors and the vestibular receptors are integrated into the solitary tract nucleus and the RVLM [5163346]. The baroreceptor reflex responds to both increases and decreases in blood pressure, however the VSR is more sensitive to decreases in blood pressure [15]. Therefore, the VSR plays an important role in the compensatory response following OH, as the vestibular receptors are highly sensitive to decreases in cerebral blood flow following sudden postural changes. In older patients whose vestibular function is weakened by aging or in patients with bilateral vestibular loss, the occurrence of OH increases [6]. As the vestibular system plays a feedforward role in postural control, there is only a short delay in the regulation of blood pressure following postural changes. However, due to the feedforward mechanism, an excessive response can occur. When vestibular function is normal, any overcorrection is largely compensated by the baroreceptor reflex [46].
Neurogenic control of the VSR leads to increased blood pressure by a direct contraction of blood vessels via the renal nerves, vasomotor nerves of muscles, and nerves innervating the limbs [1047]. It is a rapid regulatory reflex that increases blood pressure as a postural change occurs. Humoral control of the VSR provides a delayed response to postural changes by the production of epinephrine. In this study, we obtained spatial information in regards to the VSR following SNP-induced hypotension. However, the temporal correlation between pERK expression and epinephrine release could not be directly measured, due to the delayed expression of pERK following stimulation (Fig. 7).
In conclusion, SNP-induced hypotension leads to the VSR which regulates blood pressure by neurogenic and humoral control mechanism. In the neurogenic pathway, hypotension stimulates the vestibular receptors, and the excitatory signals are transmitted to the T4~7 IMC via the MVN and the RVLM. This leads to the peripheral sympathetic nerves which cause an increase in blood pressure. This pathway could be referred to as the direct response of the VSR. The humoral pathway leads to an increase in blood pressure by causing an increase in epinephrine release from the adrenal medulla. This could be referred to as the delayed response of the VSR.
Figures and Tables
 | Fig. 1The effect of intravenous injection of sodium nitroprusside (SNP) and glutamate receptor agonists on blood pressure.ACSF, AMPA, and NMDA, microinjection of artificial cerebrospinal fluid, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid, and N-methyl-D-aspartic acid, respectively, into the MVN. Intravenous injection of SNP at a dose of 15 µg/kg led to a decrease in blood pressure. Microinjection of AMPA or NMDA into the left MVN at a concentration of 1.5 mM and a volume of 10 µl led to increased blood pressure. Blood pressure did not change following the application of ACSF. Arrows indicate the microinjection of ACSF and glutamate receptor agonists. In scale bar, vertical line represents the level of blood pressure (40 mmHg) and horizontal line represents time (1 min).
|
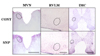 | Fig. 2Photomicrographs showing the expression of pERK in the medial vestibular nucleus (MVN), rostral ventrolateral medullary nucleus (RVLM) and intermediolateral cell column (IMC) of the T6 spinal cord, following intravenous injection of SNP.CONT, control with intravenous saline injection; SNP, intravenous SNP injection. Dotted circles represent the location of each nucleus. Scale bar=1 mm in MVN, 400 µm in RVLM and in IMC.
|
 | Fig. 3Photomicrographs showing the effect of the application of glutamate to the medial vestibular nucleus (MVN) on pERK expression in the spinal cord.(A) The effect of microinjection of glutamate receptor agonists into the left MVN on pERK expression in the intermediolateral cell column of the T6 spinal cord. (B) The effect of a microinjection of glutamate receptor antagonists into the left MVN on pERK expression in the intermediolateral cell column of the T6 spinal cord. ACSF, microinjection of artificial cerebrospinal fluid into the MVN; NMDA, microinjection of NMDA into the MVN; AMPA, microinjection of AMPA into the MVN; ACSF+SNP, SNP infusion after pretreatment with ACSF in the MVN; MK801+SNP, SNP infusion after pretreatment with MK801 in the MVN; CNQX+SNP, SNP infusion after pretreatment with CNQX in the MVN. The right lower quadrants A and B represent a higher magnification. ACSF, artificial cerebrospinal fluid; AMPA, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; IMC, intermediolateral cell column; MK801, (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d] cyclohepten-5,10-imine maleate; NMDA, N-methyl-D-aspartic acid; SNP, sodium nitroprusside. (C) Bar histogram showing the effect of the application of glutamate to the medial vestibular nucleus (MVN) on pERK expression in the spinal cord. ACSF, microinjection of artificial cerebrospinal fluid into the MVN; NMDA, microinjection of NMDA into the MVN; AMPA, microinjection of AMPA into the MVN; ACSF+SNP, SNP infusion after pretreatment with ACSF in the MVN; MK801+SNP, SNP infusion after pretreatment with MK801 in the MVN; CNQX+SNP, SNP infusion after pretreatment with CNQX in the MVN. Six animals were included in each group. Values are represented as the mean±SE. *Significant difference from ACSF group (**p<0.01). †Significant difference from ACSF+SNP group (‡p<0.01).
|
 | Fig. 4Photomicrographs showing the effect of glutamate application to the rostral ventrolateral medullary nucleus (RVLM) on pERK expression in the spinal cord.(A) The effect of a microinjection of a glutamate receptor agonist into the right RVLM on pERK expression in the intermedio-lateral cell column of the T6 spinal cord. (B) The effect of a pretreatment of a glutamate receptor antagonist into the right RVLM on pERK expression in the intermediolateral cell column of the T6 spinal cord. The right lower quadrants in A and B represent a higher magnification. Notations are as in the previous figures. (C) A bar histogram showing the effect of glutamate application to the rostral ventrolateral medullary nucleus (RVLM) on pERK expression in the spinal cord. Six animals were included in each group. Values are represented as the mean±SE. *Significant difference from ACSF group (**p<0.01). †Significant difference from ACSF+SNP group (‡p<0.01). Notations are as in the previous figures.
|
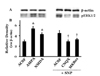 | Fig. 5Expression of pERK in the intermediolateral cell column of T6 spinal cord following microinjection of a glutamate receptor agonist or antagonist into the medial vestibular nucleus.(A) A photograph showing a Western blot for the expression of pERK from a cell membrane fraction of the intermediolateral cell column. (B) The relative density of expression was represented as the ratio between the levels of expression of pERK1/2 protein and β–actin. Six animals were included in each group. Values are represented as the mean SD. *Significant difference from ACSF group (*p<0.05). †Significant difference from ACSF+SNP group (†p<0.05).
|
 | Fig. 6The effect of glutamate on the blood levels of epinephrine.(A) The effect of microinjection of a glutamate receptor agonist or antagonist into the left medial vestibular nucleus (MVN) on levels of blood epinephrine. (B) The effect of microinjection of a glutamate receptor agonist or antagonist into the left rostral ventrolateral medullary nucleus (RVLM) on the levels of blood epinephrine. Six animals were included in each group. Values are represented as the mean±SE. *Significant difference from ACSF group (**p<0.01). †Significant difference from ACSF+SNP group (‡p<0.01). Notations are as in the previous figures.
|
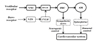 | Fig. 7A schematic diagram of the neurogenic and humoral control of the vestibulosympathetic reflex.VNC, vestibular nuclear complex; RVLM, rostral ventrolateral medullary nuclei; IMC, intermediolateral cell column of the spinal cord; AM, adrenal medulla; NTS, nucleus tractus solitarius; CVLM, caudal ventrolateral medullary nucleus.
|
Notes
References
1. Schatz IJ, Bannister R, Freeman RL, Goetz CG. The definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. J Auton Nerv Syst. 1996; 58:123–124.
2. Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002; 20:1675–1688.
3. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006; 7:335–346.
4. Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999; 519(Pt 1):1–10.
5. Jiang X, Li LW, Lan Y, Yang YZ, Jin GS, Kim MS, Park BR, Jin YZ. Comparative analysis of vestibular receptor and baroreceptor inputs to the nucleus tractus solitarius following acute hypotension in conscious rats. Neurosci Lett. 2014; 563:70–74.
6. Aoki M, Sakaida Y, Tanaka K, Mizuta K, Ito Y. Evidence for vestibular dysfunction in orthostatic hypotension. Exp Brain Res. 2012; 217:251–259.
7. Wilson VJ, Jones GM. Mammalian vestibular physiology. New York: Plenum Press;1979.
8. Park BR, Kim MS, Kim JH, Jin YZ. Effects of acute hypotension on neuronal activity in the medial vestibular nuclei of rats. Neuroreport. 2001; 12:3821–3824.
9. Yates BJ. Vestibular influences on the sympathetic nervous system. Brain Res Brain Res Rev. 1992; 17:51–59.
10. Kerman IA, Yates BJ, McAllen RM. Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R109–R117.
11. Voustianiouk A, Kaufmann H, Diedrich A, Raphan T, Biaggioni I, Macdougall H, Ogorodnikov D, Cohen B. Electrical activation of the human vestibulo-sympathetic reflex. Exp Brain Res. 2006; 171:251–261.
12. Yates BJ, Yamagata Y, Bolton PS. The ventrolateral medulla of the cat mediates vestibulosympathetic reflexes. Brain Res. 1991; 552:265–272.
13. Yates BJ, Grélot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol. 1994; 267:R974–R983.
14. Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res. 1994; 98:200–212.
15. Lan Y, Yang YZ, Jiang X, Li LW, Jin GS, Kim MS, Park BR, Jin YZ. Additive role of the vestibular end organ and baroreceptors on the regulation of blood pressure in rats. Korean J Physiol Pharmacol. 2013; 17:367–373.
16. Lan Y, Lu HJ, Jiang X, Li LW, Yang YZ, Jin GS, Park JY, Kim MS, Park BR, Jin YZ. Analysis of the baroreceptor and vestibular receptor inputs in the rostral ventrolateral medulla following hypotension in conscious rats. Korean J Physiol Pharmacol. 2015; 19:159–165.
17. Lu HJ, Li MH, Li MZ, Park SE, Kim MS, Jin YZ, Park BR. Functional connections of the vestibulo-spino-adrenal axis in the control of blood pressure via the vestibulosympathetic reflex in conscious rats. Korean J Physiol Pharmacol. 2015; 19:427–434.
18. Choi MA, Lee JH, Hwang JH, Choi SJ, Kim MS, Park BR. Signaling pathway of glutamate in the vestibular nuclei following acute hypotension in rats. Brain Res. 2008; 1229:111–117.
19. Kim MS, Choi MA, Choi DO, Lee MY, Kim KY, Rhee JK, Jin YZ, Park BR. Asymmetric activation of extracellular signal-regulated kinase 1/2 in rat vestibular nuclei by unilateral labyrinthectomy. Brain Res. 2004; 1011:238–242.
20. Morrison SF. Glutamate transmission in the rostral ventrolateral medullary sympathetic premotor pathway. Cell Mol Neurobiol. 2003; 23:761–772.
21. Springell DA, Costin NS, Pilowsky PM, Goodchild AK. Hypotension and short-term anaesthesia induce ERK1/2 phosphorylation in autonomic nuclei of the brainstem. Eur J Neurosci. 2005; 22:2257–2270.
22. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989; 29:261–265.
23. Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol. 2003; 23:597–616.
24. Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci Lett. 1994; 176:59–62.
25. Wei S, Lei M, Tong M, Ding J, Han Q, Xiao M. Acute baroreceptor unloading evokes Fos expression in anesthetized rat brain. Brain Res Bull. 2008; 76:63–69.
26. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY, USA: Academic Press;2007.
27. Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res. 1997; 7:63–76.
28. Holstein GR, Friedrich VL Jr, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience. 2011; 175:104–117.
29. Szentagothai J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950; 13:395–407.
30. Wilson VJ, Schor RH, Suzuki I, Park BR. Spatial organization of neck and vestibular reflexes acting on the forelimbs of the decerebrate cat. J Neurophysiol. 1986; 55:514–526.
31. Normand H, Etard O, Denise P. Otolithic and tonic neck receptors control of limb blood flow in humans. J Appl Physiol (1985). 1997; 82:1734–1738.
32. Biaggioni I, Costa F, Kaufmann H. Vestibular influences on autonomic cardiovascular control in humans. J Vestib Res. 1998; 8:35–41.
33. Jiang X, Lan Y, Jin YZ, Park JY, Park BG, Ameer AN, Park BR. Effect of vestibulosympathetic reflex and baroreflex on expression of pERK in the nucleus tractus solitarius following acute hypotension in conscious rats. Korean J Physiol Pharmacol. 2014; 18:353–358.
34. Doba N, Reis DJ. Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974; 34:9–18.
35. Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002; 105:956–961.
36. Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986; 78:1–5.
37. Kim MS, Hyo Kim J, Kry D, Ae Choi M, Ok Choi D, Gon Cho B, Jin YZ, Ho Lee S, Park BR. Effects of acute hypotension on expression of cFos-like protein in the vestibular nuclei of rats. Brain Res. 2003; 962:111–121.
38. Li XL, Nian B, Jin Y, Li LW, Jin GS, Kim MS, Park BR, Jin YZ. Mechanism of glutamate receptor for excitation of medial vestibular nucleus induced by acute hypotension. Brain Res. 2012; 1443:27–33.
39. Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst. 1997; 66:154–162.
40. Kerman IA, McAllen RM, Yates BJ. Patterning of sympathetic nerve activity in response to vestibular stimulation. Brain Res Bull. 2000; 53:11–16.
41. Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981; 112:195–203.
42. Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994; 71:2087–2092.
43. Holstein GR, Friedrich VL Jr, Martinelli GP. Projection neurons of the vestibulo-sympathetic reflex pathway. J Comp Neurol. 2014; 522:2053–2074.
44. Ray CA, Hume KM. Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol (1985). 1998; 84:450–453.
45. Sato T, Kawada T, Inagaki M, Shishido T, Sugimachi M, Sunagawa K. Dynamics of sympathetic baroreflex control of arterial pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2003; 285:R262–R270.
46. Gotoh TM, Fujiki N, Matsuda T, Gao S, Morita H. Roles of baroreflex and vestibulosympathetic reflex in controlling arterial blood pressure during gravitational stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R25–R30.
47. Bent LR, Bolton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2006; 174:701–711.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download