Abstract
Angiotensin II (Ang II) is metabolized from N-terminal by aminopeptidases and from C-terminal by Ang converting enzyme (ACE) to generate several truncated angiotensin peptides (Angs). The truncated Angs have different biological effects but it remains unknown whether Ang-(4-8) is an active peptide. The present study was to investigate the effects of Ang-(4-8) on hemodynamics and atrial natriuretic peptide (ANP) secretion using isolated beating rat atria. Atrial stretch caused increases in atrial contractility by 60% and in ANP secretion by 70%. Ang-(4-8) (0.01, 0.1, and 1 µM) suppressed high stretch-induced ANP secretion in a dose-dependent manner. Ang-(4-8) (0.1 µM)-induced suppression of ANP secretion was attenuated by the pretreatment with an antagonist of Ang type 1 receptor (AT1R) but not by an antagonist of AT2R or AT4R. Ang-(4-8)-induced suppression of ANP secretion was attenuated by the pretreatment with inhibitor of phospholipase (PLC), inositol triphosphate (IP3) receptor, or nonspecific protein kinase C (PKC). The potency of Ang-(4-8) to inhibit ANP secretion was similar to Ang II. However, Ang-(4-8) 10 µM caused an increased mean arterial pressure which was similar to that by 1 nM Ang II. Therefore, we suggest that Ang-(4-8) suppresses high stretch-induced ANP secretion through the AT1R and PLC/IP3/PKC pathway. Ang-(4-8) is a biologically active peptide which functions as an inhibition mechanism of ANP secretion and an increment of blood pressure.
The renin-angiotensin system (RAS) plays an important role in the regulation of body fluid and blood pressure (BP) [1]. In RAS, angiotensin II (Ang II), a major effective peptide, is rapidly metabolized to angiotensin III (Ang III), angiotensin IV (Ang IV) [123], Ang-(4-8) and Ang-(5-8) from N-terminal by the aminopeptidase A (APA), aminopeptidase N (APN), and other enzymes, respectively [23]. Ang II is also metabolized to Ang-(1-7), and Ang-(1-5) from C-terminal by angiotensin converting enzyme 2 (ACE2) and ACE, respectively [4]. Many studies are shown that the truncated Angiotensin peptides (Angs) have different effects on cellular proliferation, hemodynamics, and hormone secretions via their own receptors. Ang II type 1 receptor (AT1R) for Ang II mediates cellular hypertrophy, vasoconstriction, and increases in water intake [5], aldosterone [67] and vasopressin secretions (8), whereas AT2R for Ang II and Ang-(1-9) [3], and Mas receptor for Ang-(1-7) [9] mediate anti-hypertrophy, anti-proliferation, antifibrosis and vasodilation. Ang-(4-8) is found in the plasma of normal subjects in a very low concentration [4]. Even though Ang-(4-8) is reported to have a pressor action in normal man [10] and the antinociceptive activity [2], it is still unknown whether Ang-(4-8) is an active peptide of RAS.
On the other hand, atrial natriuretic peptide (ANP) system, as an antagonistic hormonal system of RAS, plays an important role in the regulation of BP [11]. ANP has a direct vasodilatory effect and decreased extracellular fluid (ECF) volume followed by reduction in BP [1213]. Recently, we reported that Ang II inhibits the ANP secretion via AT1R [1415] whereas Ang III [1617] and Ang IV [18] stimulate the ANP secretion via AT2R and AT4R, respectively. Ang-(1-9) [19], Ang-(1-5) [20], and Ang-(1-7) [2122] also stimulate the ANP secretion via AT2R, and Mas receptor, respectively. However, it is not known whether Ang-(4-8) plays a role in the regulation of ANP secretion. Therefore, we investigated the effects of Ang-(4-8) on hemodynamics and ANP secretion compared to Ang II and if so, defined its signaling pathway in rats.
Male Sprague-Dawley (SD) rats, purchased from Daehanbiolink (Eumsung, Korea), were housed in a temperature-controlled room with a 12:12-h light-dark cycle. Animals were provided free access to standard laboratory chow (5L79 Purina rat & mouse 18% chow, Charles River Laboratories Inc., Wilmington, MA) and water. All of experimental protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996) and were approved by our institution.
The reagents included the following: Ang-(4-8), Ang II, Losartan, PD123319, U73122, edelfosine, 2-amino-ethoxydiphenyl borate (2-APB), and staurosporine (Sigma-Aldrich, St. Louis, MO, USA), LVV-Hemorphin-7 (LVV-H7; Peptron, Daejeon, Korea), and [3H]-inulin (Perkin Elmer, Massachusetts, MA, USA).
Isolated perfused beating atria were prepared using a previously described method [23]. In brief, hearts were rapidly excised after decapitation, and left atria were dissected and inserted into cannula, and ligated by a silk. Cannulated atria were kept in an organ chamber perfused with oxygenated HEPES buffer at 37.0℃, and atria were then paced at 1.2 Hz (duration, 0.4 ms; voltage, 30 V). Intra-atrial pressure was recorded using a Power lab (ML-820, ADInstruments Pvt. Ltd, Australia) via a pressure transducer (Statham P23Db, Oxnard, CA, USA), and pulse pressure was obtained from the difference between systolic and diastolic intra-atrial pressure. The composition of HEPES buffered saline was as follows (mM): 10 HEPES, 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 10 glucose, and 0.1% bovine serum albumin (BSA). The pericardial buffered saline, which contained [3H]-inulin for measurement of translocation of extracellular fluid (ECF), was also oxygenated via silicone tube coils inside an organ chamber.
Atria were perfused for 80 min to stabilize ANP secretion and to maintain a steady-state of [3H]-inulin level in the extracellular space. Atrial perfusate was collected at intervals of 2 min at 4℃ at the condition paced at 1.2 Hz. The height, internal diameter, and slope of outflow catheter connected to atria were 5 cmH2O, 2.0 mm and 70°, respectively. To induce high atrial stretch, the height of outflow catheter was increased from 5 cmH2O to 7.5 cmH2O by connecting 2.5 cm length catheter after a 10 min control collection period and atrial perfusate was collected for 40 min [24]. The loaded volume to atria during diastole in low- and high-stretched conditions was 434 and 736 µl, respectively.
Experiments were performed with 10 groups using isolated perfused atria.
Group 1 and 2 were high-stretched atria perfused with either vehicle (n=15) or Ang-(4-8) (0.01 µM, n=10; 0.1 µM, n=10; 1 µM, n=10) [16]. After a 10 min control collection period, the height of outflow catheter was increased from 5 cmH2O to 7.5 cmH2O and Ang-(4-8) or vehicle was simultaneously perfused for 40 min [24]. Group 3, 4, and 5 were high-stretched atria perfused with Ang-(4-8) in the presence of AT1R, AT2R, or AT4R antagonist. Atria were perfused with losartan (1 µM, n=10) [14], PD123319 (1 µM, n=10) [16], or LVV-H7 (1 µM, n=10) [18] at 10 min before sample collection. After a 10 min control collection period, the height of outflow catheter was increased from 5 cmH2O to 7.5 cmH2O and Ang-(4-8) (0.1 µM) or vehicle (n=8 for each antagonist) was simultaneously perfused for 40 min [24]. Group 6, 7, 8, and 9 were high-stretched atria perfused with Ang-(4-8) in the presence of signaling pathway inhibitors. Atria were perfused with inhibitor for PLC (U73122, 10 µM, n=10; edelfosine, 1 µM, n=10), IP3 receptor (2APB, 1 µM, n=10), or PKC (staurosporine, 1 µM, n=10) [25] at 10 min before sample collection. After a 10 min control collection period, the height of outflow catheter was increased from 5 cmH2O to 7.5 cmH2O and Ang-(4-8) (0.1 µM) or vehicle (n=8 for each inhibitor) was simultaneously perfused for 40 min [24]. Group 10 was high-stretched atria perfused with Ang II (0.01, n=8; 0.1 µM, n=8) to compare to the inhibitory effect of Ang-(4-8) (0.01, n=10; 0.1 µM, n=10) on ANP secretion.
Experiments were also performed using anesthetized rats to measure changes in hemodynamics by Ang-(4-8). Male Sprague-Dawley rats, weighing 250~300 g, were anesthetized by intraperitoneal injection of mixture of ketamine and xylazine (9:1, 2 ml/kg) [16]. Body temperature was maintained at 37℃ by a heating pad. After midline incision in the neck, jugular vein and carotid artery were carefully dissected, cannulated with polyethylene tube (PE-50), and secured with ligation. The cannula in jugular vein was connected to a peristaltic pump (Minipuls 2 Gilson, Villiers le Bel, France) for infusion of Ang-(4-8) or Ang II at a constant rate of 60 µl/min [16]. The cannula in carotid artery was connected to a pressure transducer (Statham P23Db) and mean arterial pressure (MAP) and heart rate (HR) were recorded using a power lab (ML-820, ADInstruments Pvt. Ltd.). After stabilization for 10 min, various doses of Ang-(4-8) (n=5) or Ang II (n=5) were infused for 20 s and measured MAP and HR. The interval between doses was 5 min.
The concentration of ANP in perfusates and plasma was measured using a specific RIA, as described previously [26]. The intra- and inter-assay co-efficiency of variation were 6.3% (n=9) and 7.8% (n=11), respectively. The amount of secreted ANP was expressed in ng/min/g of atrial tissue. We previously reported on a two-step sequential mechanism of ANP secretion; first, the stored ANP is released from atrial myocytes into the interstitial space by atrial distension, and, second, the released ANP is secreted into the atrial lumen, concomitant with ECF translocation by atrial contraction [2728]. Therefore, the molar concentration of ANP release into the interstitium was calculated as follows:
Because the ANP secreted was found to be the processed ANP, the denominator 3063 indicates the molecular mass of ANP (1-28) (Da) [28].
Radioactivity of [3H]-inulin in atrial perfusate was measured using a liquid scintillation counter (Tris-Carb 23-TR; A Packard Bioscience Company, Downers Grove, IL) [28].
Fig. 1 shows the effects of Ang-(4-8) on high stretch-induced atrial contractility and ANP secretion with time. By high atrial stretch, atrial contractility and the secretion of atrial ANP increased significantly and maintained constantly throughout the experiments (Fig. 1A). When different doses of Ang-(4-8) (0.01 µM, 0.1 µM, and 1 µM) were perfused into atria during high atrial stretch, atrial contractility (Fig. 1Aa) and ANP secretion (Fig. 1Ab) tended to decrease.
To compare quantitatively the effects of Ang-(4-8) on high stretch-induced atrial parameters, data were recalculated by the percent change from the mean of the control period (fraction no. 1 to 5) and the peak period (fraction no. 21 to 25). Application of high atrial stretch increased atrial contractility and ANP secretion by 58.7±4.4% and 66.7±4.4%, respectively. By increasing the doses of Ang-(4-8) to 0.01, 0.1, and 1 µM, high stretch-induced ANP secretion gradually decreased from 66.7±4.4% to 46.3±6.6%, 34.6±4.3%, and 39.1±1.7%, respectively, in a dose-dependent manner (Fig. 1Bb). Even though the dose of Ang-(4-8) increased up to 10 µM, high stretch-induced ANP secretion was similar to that by 1 µM Ang-(4-8) (data not shown). Atrial pulse pressure was decreased from 58.7±4.4% to 40.4±5.5% by the highest dose of Ang-(4-8) (1 µM) (Fig. 1Ba).
To define Ang receptor subtypes related to Ang-(4-8)-induced suppression of ANP secretion, specific receptor antagonist for AT1R (losartan, 1 µM), AT2R (PD123319, 1 µM) or AT4R (LVV-H7, 1 µM) was pretreated 20 min before the administration of Ang-(4-8) or vehicle. We then compared two groups, Ang-(4-8) and vehicle groups in the presence of each antagonist. Losartan, PD123319 or LVV-H7 did not show any significant changes in atrial parameters [14151622]. Ang-(4-8) (0.1 µM) decreased ECF translocation, ANP secretion and concentration without change in atrial contractility. Pretreatment with losartan thoroughly blocked Ang-(4-8)-induced suppression of ANP secretion and concentration (Figs. 2C and 2D). However, pretreatment with PD123319 or LVV-H7 did not affect Ang-(4-8)-induced suppression of ANP secretion and concentration (Figs. 2C and 2D). The decreased ECF translocation by Ang-(4-8) was abolished by the pretreatment with losartan and LVV-H7 but not by PD123319 (Fig. 2B).
To define the downstream signaling pathway of Ang-(4-8)-induced suppression of ANP secretion through Ang receptor subtypes, several inhibitors such as PLC (U73122, 10 µM ; edelfosine, 1 µM), IP3 receptor (2APB, 1 µM) or PKC (staurosporine, 1 µM) were perfused 20 min before the administration of Ang-(4-8) or vehicle. We compared two groups, Ang-(4-8) and vehicle groups in the presence of each antagonist. Pretreatment with U73122, edelfosine, 2APB, or staurosporine attenuated Ang-(4-8)-induced suppression of ANP secretion (Fig. 3C) and ANP concentration (Fig. 3D). U73122, edelfosine, 2APB, or staurosporine did not show any significant changes in atrial parameters [14151622].
To compare Ang-(4-8)-induced suppression of ANP secretion to Ang II, atria were perfused with either Ang II (0.01, 0.1 µM) or Ang-(4-8) (0.01, 0.1 µM). Fig. 4 shows the comparison of changes in PP, ECF translocation, ANP secretion and concentration by Ang II and Ang-(4-8). Ang II (0.1 µM) decreased PP without change in ECF translocation and Ang-(4-8) (0.01 µM) decreased ECF translocation without change in PP (Fig. 4A and 4B). Both Ang II and Ang-(4-8) inhibited ANP secretion and concentration (Fig. 4C and 4D). Even though the effects of Ang-(4-8) and Ang II on the ANP concentration appear to be somewhat different at the concentration of 0.01 µM in Fig. 4D, no statistical differences in ANP secretion and concentration by Ang II and Ang-(4-8) were found.
Fig. 5 shows the comparison of hemodynamic changes by Ang-(4-8) and Ang II in anesthetized rats. Different doses of Ang II or Ang-(4-8) were infused intravenously into anaesthetized rats and BP and HR were measured. Ang II 1 nM increased MAP by 30% (Fig. 5A) and decreased HR by 7% (Fig. 5B). However, Ang-(4-8) 1 µM started to increase MAP. Ang-(4-8) 10 µM increased MAP by 27% and decreased HR by 10%.
The present study shows that Ang-(4-8) suppressed atrial ANP secretion and this effect was attenuated by the pretreatment with AT1R antagonist but not with AT2R antagonist and AT4R antagonist. Ang-(4-8)-induced suppression of ANP secretion was blocked by the pretreatment with inhibitor for PLC, IP3 receptor, and PKC. Inhibitory effect of ANP secretion by Ang-(4-8) was similar to that by Ang II but the pressure effect of Ang-(4-8) was 10,000 times less potent than Ang II. Therefore, we suggest that Ang-(4-8) suppressed high stretch-induced ANP secretion through AT1R and PLC/IP3/PKC pathway.
Ang II is metabolized to the truncated forms such as Ang III, Ang IV, Ang-(4-8), Ang-(1-9), Ang-(1-7), and Ang-(1-5), which are found in the circulation with different concentrations. Recently, many studies have been demonstrated that the truncated Angs have different effects on BP, cellular proliferation, and ANP secretion. Ang-(4-8) is found in the circulation but there are a few reports about the biological function of Ang-(4-8) [1029]. Different behavioral effects of Ang II and Ang-(4-8) have been reported in rats [29] and Ang-(5-8) has been reported to inhibit GH3 rat pituitary tumor cell proliferation independent of both MAPK p44/42 and MAPK p38 [30]. In the present study, therefore, we investigated the effects of Ang (4-8) on ANP secretion using isolated perfused rat atria. We found that Ang-(4-8) suppressed high stretch-induced ANP secretion without change in atrial contractility from isolated perfused rat atria. Ang II is metabolized to Ang-(2-8) (Ang III), Ang-(3-8) (Ang IV), and Ang-(4-8). However, Ang II inhibits ANP secretion [14] whereas Ang III and Ang IV stimulate ANP secretion in high atrial stretch condition [16]. Therefore, we compared the suppressive effect of Ang-(4-8) on ANP secretion to Ang II. Interestingly, the inhibitory potency of Ang-(4-8) on ANP secretion was similar to that of Ang II. This is the first report showing a potent inhibitory effect of Ang-(4-8) on ANP secretion similar to Ang II.
To define the receptor subtypes involving the Ang-(4-8) effect, atria were pretreated with a specific antagonist of Ang receptor. The pretreatment with AT1R antagonist attenuated Ang-(4-8)-induced suppression of ANP secretion but the pretreatment with AT2R and AT4R antagonist did not. These results suggest that the inhibitory effect of Ang-(4-8) on ANP secretion is mediated via AT1R but not via AT2R and AT4R. It has been reported that the AT1R activates PLC, leading to an increase of cytosolic Ca2+ concentration and a stimulation of PKC [31]. Activation of AT1R in pancreatic β cells also increases intracellular Ca2+ concentration by stimulating both extracellular Ca2+ influx and PLC-IP3-sensitive Ca2+ release from sarcoplasmic reticulum [32]. Therefore, in the present study, we investigated whether Ang-(4-8)-induced suppression of ANP secretion is mediated via AT1R and PLC-IP3-PKC pathway. Ang-(4-8)-induced suppression of ANP secretion was attenuated by the pretreatment with PLC inhibitor (U73122 and edelfosine). Either the inhibition of PKC (staurosporine) or IP3R (2APB) abolished the suppressive effect of Ang-(4-8) on stretch-mediated ANP secretion. This indicates IP3R-Ca2+ signaling may be an upstream signal that activates PKC, and further suggests that the Ca2+-dependent PKCs among several PKC isoforms may play a role in this signaling pathway. Therefore, we suggest that Ang-(4-8) suppressed high stretch-induced ANP secretion through AT1R and PLC/IP3/PKC pathway. These data are partly consistent with the reports showing the stimulation of the intrinsic apoptotic pathway by Ang II via AT1R/PLC/PKC pathway in cultured cardiac fibroblasts [31] and in pancreatic β cells [32].
From the above results, the suppressive effect of ANP secretion by Ang-(4-8) was similar to that by Ang II. Finally, we compared the pressor effect of Ang II with Ang-(4-8) in anesthetized normal rats. Both Ang II and Ang-(4-8) increased MAP in a dose-dependent manner. Ang II 1 nM increased MAP by 30% but Ang-(4-8) 10 µM increased MAP by 27%. Ang-(4-8) seems to be 10,000 times less potent in increasing BP than Ang II. Our data are consistent with the previous reports showing that Ang-(4-8) does elicit a minimal pressor action much less than that of Ang IV in human and rats are less responsive to Ang-(4-8) than human [33]. We do not know exactly the reason why the potency of the effects of Ang-(4-8) on ANP secretion and on blood pressure in comparison to those of Ang II shows significant discrepancy. Kono et al [33] have shown the pressor activities of Ang II, Ang III, Ang IV and Ang-(4-8) in human are approximately 100:20:0.2:0.12. Ang III and Ang-(4-8) have 10% [16] and less than 0.1% of the pressor activity of Ang II, respectively. In contrast, Ang III and Ang-(4-8) have 100% of the aldosterone secreting activity [7] and the ANP inhibiting activity of Ang II, respectively. Therefore, N-terminal amino acid of Angs seems to be more important to increase BP. Even though we did not measure the plasma concentration of Ang-(4-8) in normal condition, it has been reported that Ang-(4-8) exists in normal human blood (2.5±1.0 pM or less) [4]. The plasma concentration of Ang-(4-8) may be changed during the activation of RAS in certain pathological conditions [4], where the role of Ang-(4-8) may be expected.
In conclusion, we suggest that Ang-(4-8) suppressed high stretch-induced ANP secretion through AT1R and PLC/IP3/PKC pathway. Ang-(4-8) is a biologically active peptide which functions as an inhibition mechanism of ANP secretion rather than an increment of BP compared to Ang II.
Figures and Tables
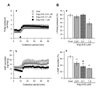 | Fig. 1Effects of angiotensin-(4-8) on atrial parameters.(A) Effects of different doses of angiotensin-(4-8) [Ang-(4-8)] (0.01, 0.1, 1.0 µM) on pulse pressure (a) and ANP secretion (b) as a function of time in isolated perfused beating atria. Atrial perfusate was collected at 2-min intervals for 50 min. Closed triangle (▲) indicates the time to increase the height of outflow catheter and to expose to Ang-(4-8). (B) Relative percent changes in pulse pressure (a) and ANP secretion (b) by different doses of Ang-(4-8) in high atrial stretch condition. Values are the mean±SEM (n=10-15). *vs. control group, p<0.05, **p<0.01; #vs. 0.01 µM Ang-(4-8) group, p<0.05; #vs. 1 µM Ang-(4-8), p<0.05.
|
 | Fig. 2Modification of effects of Ang-(4-8) on atrial parameters by receptor antagonists.Modification of effects of Ang-(4-8) (0.1 µM) on pulse pressure (A), ECF translocation (B), ANP secretion (C) and ANP concentration (D) in the presence of angiotensin receptor antagonist. Atria were pretreated with losartan (Los, 1 µM, n=10), PD123319 (1 µM, n=10), LVV-H7 (LVV, 1 µM, n=10) or vehicle (Veh, n=15) at 10 min before sample collection. After a 10 min control collection period, the height of outflow catheter was increased and Ang-(4-8) (0.1 µM) or vehicle (n=8 for each antagonist) was simultaneously perfused for 40 min. Values are the mean±SEM. *vs. each vehicle group, p<0.05, **p<0.01.
|
 | Fig. 3Modification of effects of Ang-(4-8) on atrial parameters by signaling inhibitors.Modification of effects of Ang-(4-8) (0.1 µM) on pulse pressure (A), ECF translocation (B), ANP secretion (C) and ANP concentration (D) in the presence of downstream signaling pathway inhibitors. Atria were perfused with PLC inhibitor [U73122, 10 µM, n=10; edelfosine (Edelfo), 1 µM, n=10], IP3 receptor inhibitor (2APB, 1 µM, n=10) or PKC inhibitor [staurosporine (Stauro), 1 µM, n=10] at 10 min before sample collection. After a 10 min control collection period, the height of outflow catheter was increased and Ang-(4-8) or vehicle was simultaneously perfused for 40 min. To rule out self-effects of inhibitors, atria were perfused with inhibitor alone (n=8 for each inhibitor). Values are the mean±SEM. *vs. each vehicle group, p<0.05, **p<0.01.
|
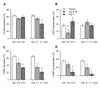 | Fig. 4Comparison of effects of Ang II and Ang-(4-8) on atrial parameters.Comparison of effects of Ang II (0.01, 0.1 µM, n=8) and Ang-(4-8) (0.01, 0.1 µM, n=10) on pulse pressure (A), ECF translocation (B), ANP secretion (C), and ANP concentration (D). Values are expressed as percent change compared to control value. Values are the mean±SEM. *vs. each vehicle group, p<0.05, **p<0.01; #vs. Ang II group, p<0.05.
|
 | Fig. 5Comparison of effects of Ang II and Ang-(4-8) on hemodynamics.(A) Representative tracing of change in arterial pressure by Ang II and Ang-(4-8) in anesthetized rats. (B) Comparison of changes in mean arterial pressure and heart rate by Ang-(4-8) (n=5) and Ang II (n=5). Values are expressed as percent change compared to control value. Values are the mean±SEM. **vs. Ang-(4-8) group, p<0.01.
|
ACKNOWLEDGEMENTS
This work was also partly supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No 2008-0062279) and (NO 2017-R1A2B-4002214).
Notes
References
1. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003; 24:261–271.
2. Guethe LM, Pelegrini-da-Silva A, Borelli KG, Juliano MA, Pelosi GG, Pesquero JB, Silva CL, Corrêa FM, Murad F, Prado WA, Martins AR. Angiotensin (5-8) modulates nociception at the rat periaqueductal gray via the NO-sGC pathway and an endogenous opioid. Neuroscience. 2013; 231:315–327.
3. Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008; 264:224–236.
4. Semple PF, Boyd AS, Dawes PM, Morton JJ. Angiotensin II and its heptapeptide (2-8), hexapeptide (3-8), and pentapeptide (4-8) metabolites in arterial and venous blood of man. Circ Res. 1976; 39:671–678.
5. Wilson WL, Roques BP, Llorens-Cortes C, Speth RC, Harding JW, Wright JW. Roles of brain angiotensins II and III in thirst and sodium appetite. Brain Res. 2005; 1060:108–117.
6. Campbell WB, Brooks SN, Pettinger WA. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science. 1974; 184:994–996.
7. Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011; 152:1582–1588.
8. Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci U S A. 1996; 93:11968–11973.
9. Bader M. ACE2, angiotensin-(1–7), and Mas: the other side of the coin. Pflugers Arch. 2013; 465:79–85.
10. Kono T, Oseko F, Ikeda F, Nakano R, Taniguchi A, Imura H, Khosla MC. Biological activity of des-(Asp1, Arg2, Val3)-angiotensin II in man. Life Sci. 1983; 32:337–343.
11. McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005; 16:469–477.
12. Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. 2005; 68:8–17.
13. Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006; 27:47–72.
14. Oh YB, Gao S, Shah A, Kim JH, Park WH, Kim SH. Endogenous angiotensin II suppresses stretch-induced ANP secretion via AT1 receptor pathway. Peptides. 2011; 32:374–381.
15. Oh YB, Gao S, Lim JM, Kim HT, Park BH, Kim SH. Caveolae are essential for angiotensin II type 1 receptor-mediated ANP secretion. Peptides. 2011; 32:1422–1430.
16. Park BM, Oh YB, Gao S, Cha SA, Kang KP, Kim SH. Angiotensin III stimulates high stretch-induced ANP secretion via angiotensin type 2 receptor. Peptides. 2013; 42:131–137.
17. Park BM, Gao S, Cha SA, Park BH, Kim SH. Cardioprotective effects of angiotensin III against ischemic injury via the AT2 receptor and KATP channels. Physiol Rep. 2013; 1:e00151.
18. Park BM, Cha SA, Lee SH, Kim SH. Angiotensin IV protects cardiac reperfusion injury by inhibiting apoptosis and inflammation via AT4R in rats. Peptides. 2016; 79:66–74.
19. Cha SA, Park BM, Gao S, Kim SH. Stimulation of ANP by angiotensin-(1-9) via the angiotensin type 2 receptor. Life Sci. 2013; 93:934–940.
20. Yu L, Yuan K, Phuong HT, Park BM, Kim SH. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides. 2016; 86:33–41.
21. Shah A, Oh YB, Shan G, Song CH, Park BH, Kim SH. Angiotensin-(1-7) attenuates hyposmolarity-induced ANP secretion via the Na+-K+ pump. Peptides. 2010; 31:1779–1785.
22. Shah A, Gul R, Yuan K, Gao S, Oh YB, Kim UH, Kim SH. Angiotensin-(1-7) stimulates high atrial pacing-induced ANP secretion via Mas/PI3-kinase/Akt axis and Na+/H+ exchanger. Am J Physiol Heart Circ Physiol. 2010; 298:H1365–H1374.
23. Yuan K, Cao C, Han JH, Kim SZ, Kim SH. Adenosine-stimulated atrial natriuretic peptide release through A1 receptor subtype. Hypertension. 2005; 46:1381–1387.
24. Han JH, Bai GY, Park JH, Yuan K, Park WH, Kim SZ, Kim SH. Regulation of stretch-activated ANP secretion by chloride channels. Peptides. 2008; 29:613–621.
25. Cui X, Wen JF, Jin JY, Xu WX, Kim SZ, Kim SH, Lee HS, Cho KW. Protein kinase-dependent and Ca2+-independent cAMP inhibition of ANP release in beating rabbit atria. Am J Physiol Regul Integr Comp Physiol. 2002; 282:R1477–R1489.
26. Cho KW, Seul KH, Ryu H, Kim SH, Koh GY. Characteristics of distension-induced release of immunoreactive atrial natriuretic peptide in isolated perfused rabbit atria. Regul Pept. 1988; 22:333–345.
27. Cho KW, Lee SJ, Wen JF, Kim SH, Seul KH, Lee HS. Mechanical control of extracellular space in rabbit atria: an intimate modulator of the translocation of extracellular fluid and released atrial natriuretic peptide. Exp Physiol. 2002; 87:185–194.
28. Cho KW, Kim SH, Hwang YH, Seul KH. Extracellular fluid translocation in perfused rabbit atria: implication in control of atrial natriuretic peptide secretion. J Physiol. 1993; 468:591–607.
29. Braszko JJ, WŁasienko J, Kupryszewski G, Witczuk B, Wisniewski K. Behavioral effects of angiotensin II and angiotensin II-(4-8)-pentapeptide in rats. Physiol Behav. 1988; 44:327–332.
30. Ptasinska-Wnuk D, Mucha SA, Lawnicka H, Fryczak J, Kunert-Radek J, Pawlikowski M, Stepien H. The effects of angiotensin peptides and angiotensin receptor antagonists on the cell growth and angiogenic activity of GH3 lactosomatotroph cells in vitro. Endocrine. 2012; 42:88–96.
31. Vivar R, Soto C, Copaja M, Mateluna F, Aranguiz P, Muñoz JP, Chiong M, Garcia L, Letelier A, Thomas WG, Lavandero S, Díaz-Araya G. Phospholipase C/protein kinase C pathway mediates angiotensin II-dependent apoptosis in neonatal rat cardiac fibroblasts expressing AT1 receptor. J Cardiovasc Pharmacol. 2008; 52:184–190.
32. Ramracheya RD, Muller DS, Wu Y, Whitehouse BJ, Huang GC, Amiel SA, Karalliedde J, Viberti G, Jones PM, Persaud SJ. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia. 2006; 49:321–331.
33. Kono T, Taniguchi A, Imura H, Oseko F, Khosla MC. Biological activities of angiotensin II-(1-6)-hexapeptide and angiotensin II-(1-7)-heptapeptide in man. Life Sci. 1986; 38:1515–1519.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download