This article has been corrected. See "Corrigendum to: Chronic cerebral hypoperfusion and plasticity of the posterior cerebral artery following permanent bilateral common carotid artery occlusion" in Volume 26 on page 557.
Abstract
Vascular dementia (VaD) is a group of heterogeneous diseases with the common feature of cerebral hypoperfusion. To identify key factors contributing to VaD pathophysiology, we performed a detailed comparison of Wistar and Sprague–Dawley (SD) rats subjected to permanent bilateral common carotid artery occlusion (BCCAo). Eight-week old male Wistar and SD rats underwent BCCAo, followed by a reference memory test using a five-radial arm maze with tactile cues. Continuous monitoring of cerebral blood flow (CBF) was performed with a laser Doppler perfusion imaging (LDPI) system. A separate cohort of animals was sacrificed for evaluation of the brain vasculature and white matter damage after BCCAo. We found reference memory impairment in Wistar rats, but not in SD rats. Moreover, our LDPI system revealed that Wistar rats had significant hypoperfusion in the brain region supplied by the posterior cerebral artery (PCA). Furthermore, Wistar rats showed more profound CBF reduction in the forebrain region than did SD rats. Post-mortem analysis of brain vasculature demonstrated greater PCA plasticity at all time points after BCCAo in Wistar rats. Finally, we confirmed white matter rarefaction that was only observed in Wistar rats. Our studies show a comprehensive and dynamic CBF status after BCCAo in Wistar rats in addition to severe PCA dolichoectasia, which correlated well with white matter lesion and memory decline.
Vascular dementia (VaD) is one of the prevalent neurological diseases among the aged population, accounting for 17.4% of all dementia patients in the US [1]. It is a heterogeneous group of diseases with different vascular mechanisms that share reduced cerebral blood flow (CBF) as a major contributor to cognitive decline [23]. Current treatment options merely try to slow VaDassociated cognitive decline, rather than cure the disease [3]. One of the main barriers to overcoming VaD is a poor understanding of the basic mechanisms underlying VaD pathogenesis. VaD is often accompanied by hypoperfusion caused by ischemia, hemorrhage, or small vessel disease [34]. Under conditions of chronic hypoperfusion, white matter rarefaction, glial activation, and axon damage can characteristically develop [567]. Although many experimental studies have tried to identify critical players contributing to VaD pathophysiology, it is still not clear how these factors are altered during the course of disease progression and how they are interactive in the longitudinal direction.
To recapitulate the neurological and behavioral changes observed in VaD, various animal models have been used including chronic or transient global hypoperfusion, focal hypoperfusion, embolic occlusion, or hypertensive animals [8]. Among them, permanent bilateral occlusion of common carotid arteries (BCCAo) in rodents is a reliable model for investigation of the cognitive and histopathologic consequences of chronic cerebral hypoperfusion [389]. Rats are a suitable species for inducing chronic global hypoperfusion because their complete circle of Willis affords constant blood supply to the forebrain after the onset of BCCAo [9]. Wistar and Sprague–Dawley (SD) rats are the two most frequently chosen strains, but their histologic findings after BCCAo appear to be different: white matter lesions prominently appear in Wistar rats, while hippocampal neuronal death is only detected in SD rats [1011]. Therefore, in the present study, we sought to determine critical features in VaD pathophysiology by comparing these two representative rat strains after BCCAo.
All animal procedures were approved by the Ethics Committee of the Catholic University of Korea and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Chronic cerebral hypoperfusion was induced in male Wistar-ST rats (Joongang Lab, Seoul, Korea) and male SD rats (Koatech, Kyungki-do, Korea) weighing 250~270 g, as previously described with minor modifications [5]. Briefly, after induction with 4% halothane, rats were anesthetized with 1.5% halothane in a 70% nitrous oxide and 30% oxygen mixture using a face mask; anesthesia was continued throughout the surgical procedure. After making a midline incision, both common carotid arteries were carefully exposed without damaging the vagus nerve. The carotid arteries were double ligated using silk sutures, trying to minimize injuries of the vessels and adjacent tissues. With the exception of occlusion of the carotid arteries, surgical procedures in sham-operated animals were the same as those in the BCCAo-operated animals. During surgery, rectal temperature was maintained at 37.0~37.9℃ with a heating pad. After the operation, all animals were returned to their cages with free access to food and water. On the 7th, 14th, and 21st days after the surgery (BCCAo or sham operation), the animals were euthanized with 15% chloral hydrate.
Reference memory was assessed using the five-radial arm maze with different tactile cues. To encourage reward-seeking behavior, food was gradually restricted to 85% of the initial weight. SD and Wistar rats were first placed in the maze where small pieces of food pellets were scattered in all five arms with different tactile cues to become familiarized with the radial arm maze for 30 min. On subsequent days, food was placed only at the ends of the 2nd and 4th arms, and the animals were given two training sessions per day for 5 consecutive days. One rat at a time was placed in the central zone of the maze. After 1 min, all guillotine doors were opened, and the animal was allowed to enter the arms for 10 min to receive a food reward. To eliminate scent trails, the maze was wiped with 70% alcohol after each trial session. The total number of entries into the arms without a food pellet and entries into the arms with food but no ingestion was divided by the total number of arms visited to give the error rate. Only rats showing error rates lower than 5% were selected and further subjected to sham or BCCAo operations. After 7 days of recovery following BCCAo, the animal was placed for 5 min in the maze where a food reward was located in the 2nd and 4th arms, which was exactly the same as the training sessions. For 10 consecutive days, the number of incorrect arm choices, i.e., entering the arms without a reward or entering the arms with a reward but no intake, was evaluated to test the reference memory of SD or Wistar rats that underwent sham- and BCCAo-manipulation, as BCCAo does not induce motor deficits in general [912].
To monitor cortical blood flow in the whole brain, we used a Periscan PIM 3 Laser Doppler Perfusion Imager (LDPI; PIM3, Perimed, Järfälla, Sweden). The LDPI system uses a low-power laser light that is scattered in the tissue and detects changes in wavelength when the light hits moving blood cells in the cortical blood vessels. For efficient transfer of the laser beam to the cortex, the skull surface was thinned using a hand drill. The distance between the LDPI scanner head and the skull was 6.5 cm, producing high-resolution LDPI images with a 99×85 pixel matrix. Cortical microperfusion was repetitively scanned before and at 30 min, 7 days, 14 days, and 21 days after BCCAo and was presented as color-coded images. The blood flow values of the middle cerebral artery (MCA) and the posterior cerebral artery (PCA) territories were numerically determined by the concentration of moving blood cells and mean velocity of these blood cells, which was calculated from the magnitude of the Doppler signal and the frequency shift. Finally, data were presented as % CBF based on the value collected immediately before BCCAo or sham manipulation.
At each time point after sham or BCCAo, animals were transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brain vasculature was visualized with the latex-perfusion technique for cerebral vessels [10]. The whole brain was photographed with a digital camera (EOS 300D; Canon, Tokyo, Japan), followed by post-fixation in 4% paraformaldehyde for 4 h. The degree of plasticity of the PcomA and PCA was evaluated by comparing the diameter with that of the basilar artery using Image-Pro Plus software version 5.1 (MediaCybernetics, Silver Spring, MD, USA), represented as a relative plasticity of PcomA and PCA.
White matter lesions were evaluated by Luxol fast blue staining in regions located −2.64 to −3.12 mm from bregma [5]. After post-fixation, the brains were dehydrated with 30% sucrose and embedded in Tissue-Tek (Sakura Finetechnical, Tokyo, Japan). Tissue sections (20 µm) were mounted on the slide and incubated with Luxol fast blue (Sigma, St. Louis, MO, USA) at 56℃ overnight. Next, the slides were sequentially soaked with 0.05% lithium carbonate solution, distilled water, and 70% ethanol. Finally, the slides were dehydrated in 100% ethanol, cleared in xylene, and coverslipped. White matter damage was evaluated in a double-blind manner, and three sections per animal were analyzed. The severity of white matter rarefaction was graded as normal (grade 0), disarrangement of nerve fibers (grade 1), formation of marked vacuoles (grade 2), and disappearance of myelinated fibers (grade 3).
All data are presented as mean±SEM. SPSS software (version 21.0, IBM SPSS Corp.) was used for statistical comparison. Differences in reference memory and CBF among four different groups were determined by repeated measures analysis of variance (ANOVA) with Duncan's post hoc mean comparison. PCA and PcomA plasticity were analyzed using one-way ANOVA, followed by Duncan's post hoc test. Fold change of relative PCA plasticity based on the sham controls was determined by two-tailed Student's t test with assumption of normal distribution. Finally, white matter damage was analyzed by Pearson Chi-square test. p<0.05 was considered statistically significant.
To test reference memory impairment after BCCAo, SD and Wistar rats were pre-trained for 5 days to remember the arms with a food reward. For 10 consecutive days from the 8th day after BCCAo, we placed the rats in the five-radial arm maze once a day and evaluated whether they could acquire the reward (Figs. 1A, 1C). Because of white matter damage in the optic tract, different tactile cues were provided at each arm to help blinded rats find the correct arms (Fig. 1B). Entry into the arms without a food pellet (wrong arm) and entry into the food-containing arms but leaving the food uneaten were defined as incorrect choices (Fig. 1C). SD rats made a similar number of errors at all times assessed regardless of hypoperfusion injury, demonstrating that SD animals in both sham and BCCAo groups maintained reward-related reference memory (Fig. 1D). However, Wistar rats showed a higher number of incorrect choices after BCCAo compared with sham-treated animals, suggesting reference memory deficits induced by BCCAo (Fig. 1D).
To explain why the BCCAo procedure resulted in cognitive dysfunction in the Wistar strain but not in SD rats, we monitored cortical microperfusion under the entire skull for 3 weeks. Tracking temporal CBF in the same animal could provide integrated views of CBF alterations after BCCAo (Fig. 2A). Forebrain perfusion examined at the MCA territory was markedly reduced immediately after BCCAo in both SD and Wistar rats (Fig. 2B). Moreover, over the subsequent 21 days, the cortical blood flow of Wistar and SD rats was lower after BCCAo than in sham-manipulated animals, as analyzed by repeated measures of ANOVA. However, in PCA territory, Wistar rats remained hypoperfused after BCCAo, while SD rats recovered CBF similar to that of sham-operated animals (Fig. 2C). Compared with the SD strain, Wistar rats showed significantly lower CBF in both MCA and PCA territories after BCCAo (Figs. 2B, 2C). In summary, in MCA territory, BCCAo induced hypoperfusion in both SD and Wistar rats, with more profound CBF reduction in Wistar rats. In contrast, in PCA territory, only Wistar rats showed low CBF. These data suggest that posterior circulation is a critical factor in the difference in BCCAo-induced hypoperfusion between SD and Wistar rats.
Considering that Wistar rats demonstrated memory deficits and hypoperfusion in PCA territory after BCCAo, which were not observed in SD rats, we decided to examine brain vasculature. The latex-perfusion technique for cerebral vessels was applied at 7, 14, and 21 days after BCCAo or sham-manipulation. In sham-operated animals, significant differences in the plasticity of the PcomA and the PCA were found between SD and Wistar rats (Figs. 3A, 3B). The ratio of the diameter of the PcomA to that of the basilar artery in SD and Wistar rats was 0.84±0.05 and 0.64±0.11, respectively (Fig. 3B). In addition, relative PCA diameter in Wistar rats was clearly smaller than that of SD rats (Fig. 3B). Although sham manipulation may affect the plasticity of these vessels, however, as we tried to minimize unnecessary dissections of the common carotid arteries and the adjacent tissues, it is likely that SD and Wistar rats have a basal difference in the diameter of PcomA and PCA. After BCCAo, no significant difference was observed in plasticity of the PcomA in SD or Wistar rats during the course of the study (Figs. 3A, 3B). However, the relative diameter of PCA was markedly increased in both SD and Wistar rats at all time points after BCCAo (Figs. 3A, 3B). Notably, the fold change in PCA plasticity based on the sham value was significantly greater in Wistar than SD rats (Fig. 3C), suggesting that more reactive changes occurred in the vascular system of Wistar rats.
To determine whether chronic cerebral hypoperfusion and vascular plasticity can be translated into histological changes, we performed Klüver-Barrera luxol fast blue myelin staining (Fig. 4). After 21 days, sham-operated SD and Wistar rats demonstrated an intact optic tract, which was chosen as one of the most vulnerable regions to white matter damage (Fig. 4A). Interestingly, prominent vacuolation and serpentine white matter fibers were observed in Wistar rats after BCCAo, whereas SD rats showed almost intact myelin fibers (Fig. 4A). Moreover, Chi-square statistical analysis revealed that BCCAo significantly induced white matter lesions in Wistar rats compared with SD rats (Fig. 4B).
Defining key changes after experimental BCCAo is critical for a better understanding of the basic mechanisms of VaD and thus for the development of effective therapeutic strategies. Our data showed a clear contrast in memory function between Wistar and SD rats after BCCAo. To gain mechanistic insight, we performed repetitive cortical blood flow monitoring using a LDPI system. BCCAo induced profound reduction in CBF in the forebrain region over the course of chronic hypoperfusion in both Wistar and SD rats. Interestingly, CBF in PCA territory was significantly reduced after BCCAo in Wistar rats, but not in SD rats. These findings led us to investigate structural plasticity of the brain vasculature, which showed greater PCA plasticity in Wistar rats after BCCAo. We also confirmed histologic white matter damage in Wistar rats that was not observed in SD rats, in line with our previous report [11].
As learning and memory impairment is a central feature of VaD, extensive efforts have been made to show different aspects of cognitive decline using a variety of behavioral tests in experimental models [8]. When focusing on reference memory function, the Wistar rat is the major strain showing reduced reference memory after BCCAo [13141516171819], although a few studies have reported memory deficits in SD rats [2021]. In our study, Wistar rats demonstrated a larger number of errors in the five-radial arm maze test when they were required to recall previous memory from 1 week after BCCAo, consistent with other reports [1419]. Moreover, we carefully adopted tactile cues for the radial arm maze test to exclude the possible confounding factor of BCCAo-induced blindness in Wistar rats, as visual guidance is essential in both the Morris water maze and the classic radial arm maze test. We believe this approach allows us to evaluate BCCAo-induced memory dysfunction in Wistar rats in the most accurate way, making our findings highly credible. Interestingly, SD rats showed no memory deficits at the second week following BCCAo. Previous studies reported poor reference memory in SD rats at about 2 months after injury [2021]. Moreover, reference memory was not reduced until 3 weeks post-BCCAo [20], supporting our findings. As the onset of reference memory dysfunction appeared to be delayed in the SD strain compared to Wistar rats, future studies are warranted to investigate which factors play crucial roles in VaD pathophysiology in the chronic stages after global hypoperfusion.
Since we found apparent differences in reference memory function between Wistar and SD rats after BCCAo, we assessed temporal CBF alteration as a next step. Although many studies reported CBF reduction after BCCAo using various tools including radioisotope techniques, microsphere injection, laser Doppler flowmetry, and magnetic resonance imaging [610141522232425], each of these methods has intrinsic limitations such as poor accuracy of measurement or lack of consideration of multiple brain regions. Thus, differential hemodynamic evaluation remains an open question to be addressed with better spatial and temporal resolution. Here, we showed BCCAo-induced CBF fluctuation using the LDPI system. This system enabled us to monitor real-time CBF perturbation after BCCAo non-invasively and repetitively in the same animals. Moreover, scanning speckles derived from the motion of moving blood cells under the entire skull surface could provide a general overview of alterations in cortical microcirculation induced by chronic hypoperfusion. Indeed, side-by-side comparison of temporal CBF fluctuation between Wistar and SD rats uncovered a marked reduction in posterior circulation in only the Wistar rats after BCCAo, in addition to a more profound CBF decrease in MCA territory compared with SD rats. These findings can help to reconstruct the comprehensive CBF status after BCCAo in a temporal axis, further permitting a better understanding of the pathologic progression of VaD.
Dolichoectasia refers to dilated and elongated blood vessels. Vertebrobasilar dolichoectasia is commonly reported in the aged population that is vulnerable to VaD [2627]. Supporting this phenotype, experimental VaD studies showed increased length and tortuosity of the basilar artery [622], which we also noticed in our study. As BCCAo increased the diameter of many other blood vessels, including PCA and PcomA [22], we focused on the plasticity of these vessels relative to the basilar artery. Not surprisingly, PCA plasticity was increased after BCCAo in both SD and Wistar rats. However, the degree of PCA plasticity after BCCAo was significantly greater in Wistar rats at all time points examined. Reactive vascular ectasia can be one of the compensatory mechanisms induced by BCCAo, but it fails to be translated into CBF restoration demonstrated by LDPI monitoring in PCA territory. Considering these findings, together with cognitive dysfunction and white matter damage in Wistar rats, severe PCA dolichoectasia could be a novel screening marker for the diagnosis of VaD. Clinical data nicely support this hypothesis as intracranial arterial dolichoectasia in stroke patients was highly correlated with the incidence of lacunar infarction and small-vessel diseases [282930], possibly leading to VaD [31]. Thus, it will be interesting to further examine the causative link between the severity of PCA dolichoectasia and the functional and cognitive status of VaD.
Diagnosis and treatment of VaD have been challenging because of the heterogeneous nature of the disease, which can result in pleiotropic manifestations of VaD pathology. The first step in overcoming this obstacle will be the identification of major factors contributing to VaD pathogenesis. In this paper, by analyzing two representative rat strains subjected to BCCAo, we showed severe PCA dolichoectasia and marked hypoperfusion in the PCA-supplying region in Wistar rats. Moreover, these findings were associated with white matter lesions and reference memory impairment. Since our studies provide a comprehensive examination of BCCAo-induced CBF perturbation and unique vascular plasticity, our results could lead to the development of surrogate markers of VaD progression.
Figures and Tables
 | Fig. 1Reference memory function after bilateral common carotid artery occlusion (BCCAo) assessed using a five-radial arm maze with tactile cues.(A) Time line showing the experimental design. Before undergoing BCCAo, SD and Wistar rats were trained to remember the arms with a food pellet. From the 8th day post-BCCAo, the ability of SD and Wistar rats to find the food-placed arms was tested over 10 days. (B) Representative image of different tactile cues applied for radial arm maze. (C) Representative image of a five-radial arm maze apparatus. The yellow box refers to the location of the food pellet, which is indicated as a red star. (D) Graph showing the number of errors during the memory test period. Wistar rats subjected to BCCAo (n=6) demonstrated a significantly higher number of incorrect choices compared with sham-treated Wistar (n=9), sham-operated SD (n=9), and BCCAo-treated SD (n=8) rats. Data are given as mean±SEM. *p<0.05 by repeated measures analysis of variance with Duncan's post hoc mean comparison. SD, Sprague–Dawley; SD sham, SD rats subjected to sham operation; SD BCCAo, SD rats subjected to BCCAO; Wistar sham, Wistar rats subjected to sham operation; Wistar BCCAo, Wistar rats subjected to BCCAo.
|
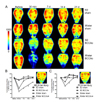 | Fig. 2Longitudinal whole-brain monitoring of cerebral blood flow (CBF) after bilateral common carotid artery occlusion (BCCAo) in SD and Wistar rats.(A) Representative color-coded examples of serial laser Doppler perfusion images. Cortical microperfusion of Wistar and SD rats subjected to sham or BCCAo was repetitively monitored using a laser Doppler perfusion imaging system. (B) Graph showing CBF perturbation in MCA territory, indicated as two circles in the right image. After BCCAo, Wistar rats (n=7) and SD rats (n=9) showed a marked CBF reduction compared with sham-treated Wistar (n=7) and SD (n=8) rats. Notably, cerebral hypoperfusion in MCA territory was significantly greater in Wistar rats than SD rats. Data are given as mean±SEM. *p<0.05 compared to sham and †p<0.05 compared to Wistar rats subjected to BCCAo. Repeated measures analysis of variance with Duncan's post hoc mean comparison was performed. (C) Graph showing CBF perturbation in PCA territory, indicated as a circle in the right image. Wistar rats after BCCAo (n=7) showed marked CBF reduction compared to sham-treated Wistar (n=7), sham-treated SD (n=8), and BCCAo-operated SD (n=9) rats. Data are given as mean±SEM. *p<0.05 by repeated measures analysis of variance with Duncan's post hoc mean comparison. SD, Sprague–Dawley; SD sham, SD rats subjected to sham operation; SD BCCAo, SD rats subjected to BCCAO; Wistar sham, Wistar rats subjected to sham operation; Wistar BCCAo, Wistar rats subjected to BCCAo; MCA, middle cerebral artery territory; PCA, posterior cerebral artery.
|
 | Fig. 3Structural plasticity of the brain vasculature at the circle of Willis in SD and Wistar rats after bilateral common carotid artery occlusion (BCCAo).(A) Representative images of major blood vessels at 7 days (SD: n=7, Wistar: n=9), 14 days (SD: n=7, Wistar: n=6), and 21 days (SD: n=7, Wistar: n=8) after BCCAo or sham operation (SD: n=12, Wistar: n=7). (B) Table showing the ratio of the posterior communicating artery (PcomA) and posterior cerebral artery (PCA) diameter to that of the basilar artery in SD and Wistar rats. The relative PcomA and PCA were significantly thinner in Wistar rats than in SD rats. One-way analysis of variance also demonstrated that PCA plasticity was significantly increased at all time points after BCCAo compared with sham values for both SD and Wistar strains. Values are mean±SEM. †p<0.05: SD sham vs. Wistar sham; *p<0.05: sham vs. the rest of the time points assessed by one-way analysis of variance and Duncan's post hoc test. (C) Graph showing the fold change of relative PCA plasticity based on the sham-controls. Wistar rats showed significantly greater PCA plasticity than SD rats at all time points examined. *p<0.05 by two-tailed Student's t test. Values are mean±SEM. SD, Sprague–Dawley; MCA, middle cerebral artery; ACA, anterior cerebral artery; ICA, internal carotid artery; BA, basilar artery.
|
 | Fig. 4White matter rarefaction in SD and Wistar rats.(A) Representative microscopic images of the optic tract stained by Klüver-Barrera Luxol fast blue. Only Wistar rats subjected to bilateral common carotid artery occlusion (BCCAo) showed prominent vacuolation and serpentine white matter fibers, indicative of white matter damage. Scale bar in SD sham=50 µm (valid for all the other images). (B) Graph showing the severity of white matter lesion. Semi-quantitative analysis of white matter rarefaction showed a significantly higher grade of white matter damage after BCCAo in Wistar rats (n=7) compared with SD rats (n=8). Data are given as mean±SEM. *p<0.05 by Pearson Chi-square test. SD, Sprague–Dawley.
|
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2017R1A2B4002704 and NRF-2014R1A1A3049456).
Notes
References
1. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007; 29:125–132.
2. Sopala M, Danysz W. Chronic cerebral hypoperfusion in the rat enhances age-related deficits in spatial memory. J Neural Transm (Vienna). 2001; 108:1445–1456.
3. Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015; 272:97–108.
4. Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, López-Pousa S, Arizaga R, Wallin A. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004; 226:81–87.
5. Cho KO, La HO, Cho YJ, Sung KW, Kim SY. Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res. 2006; 83:285–291.
6. Soria G, Tudela R, Márquez-Martín A, Camón L, Batalle D, Muñoz-Moreno E, Eixarch E, Puig J, Pedraza S, Vila E, Prats-Galino A, Planas AM. The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One. 2013; 8:e74631.
7. Wakita H, Tomimoto H, Akiguchi I, Kimura J. Glial activation and white matter changes in the rat brain induced by chronic cerebral hypoperfusion: an immunohistochemical study. Acta Neuropathol. 1994; 87:484–492.
8. Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem. 2010; 115:814–828.
9. Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007; 54:162–180.
10. Kim SK, Cho KO, Kim SY. The plasticity of posterior communicating artery influences on the outcome of white matter injury induced by chronic cerebral hypoperfusion in rats. Neurol Res. 2009; 31:245–250.
11. Kim SK, Cho KO, Kim SY. White matter damage and hippocampal neurodegeneration induced by permanent bilateral occlusion of common carotid artery in the rat: comparison between wistar and sprague-dawley strain. Korean J Physiol Pharmacol. 2008; 12:89–94.
12. Tanaka K, Wada N, Hori K, Asanuma M, Nomura M, Ogawa N. Chronic cerebral hypoperfusion disrupts discriminative behavior in acquired-learning rats. J Neurosci Methods. 1998; 84:63–68.
13. Storozheva ZI, Proshin AT, Sherstnev VV, Storozhevykh TP, Senilova YE, Persiyantseva NA, Pinelis VG, Semenova NA, Zakharova EI, Pomytkin IA. Dicholine salt of succinic acid, a neuronal insulin sensitizer, ameliorates cognitive deficits in rodent models of normal aging, chronic cerebral hypoperfusion, and beta-amyloid peptide-(25-35)-induced amnesia. BMC Pharmacol. 2008; 8:1.
14. Ohta H, Nishikawa H, Kimura H, Anayama H, Miyamoto M. Chronic cerebral hypoperfusion by permanent internal carotid ligation produces learning impairment without brain damage in rats. Neuroscience. 1997; 79:1039–1050.
15. Zhang ZH, Shi GX, Li QQ, Wang YJ, Li P, Zhao JX, Yang JW, Liu CZ. Comparison of cognitive performance between two rat models of vascular dementia. Int J Neurosci. 2014; 124:818–823.
16. Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005; 139:169–177.
17. Farkas E, Institóris A, Domoki F, Mihály A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusionrelated learning dysfunction and brain damage after carotid artery occlusion. Brain Res. 2004; 1008:252–260.
18. Ma X, Sun Z, Liu Y, Jia Y, Zhang B, Zhang J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen Res. 2013; 8:2050–2059.
19. Ni J, Ohta H, Matsumoto K, Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfusion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 1994; 653:231–236.
20. Pappas BA, de la Torre JC, Davidson CM, Keyes MT, Fortin T. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 1996; 708:50–58.
21. Li WZ, Wu WY, Huang H, Wu YY, Yin YY. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol Med Rep. 2013; 8:935–941.
22. Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, Lythgoe MF. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab. 2006; 26:1066–1075.
23. Tsuchiya M, Sako K, Yura S, Yonemasu Y. Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res. 1992; 89:87–92.
24. Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol. 2003; 30:266–272.
25. Ulrich PT, Kroppenstedt S, Heimann A, Kempski O. Laser-Doppler scanning of local cerebral blood flow and reserve capacity and testing of motor and memory functions in a chronic 2-vessel occlusion model in rats. Stroke. 1998; 29:2412–2420.
26. Smoker WR, Corbett JJ, Gentry LR, Keyes WD, Price MJ, McKusker S. High-resolution computed tomography of the basilar artery: 2. Vertebrobasilar dolichoectasia: clinical-pathologic correlation and review. AJNR Am J Neuroradiol. 1986; 7:61–72.
27. Lin YW, Chen CH, Lai ML. The dilemma of treating vertebrobasilar dolichoectasia. Clin Pract. 2012; 2:e84.
28. Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol. 2005; 57:472–479.
29. Ince B, Petty GW, Brown RD Jr, Chu CP, Sicks JD, Whisnant JP. Dolichoectasia of the intracranial arteries in patients with first ischemic stroke: a population-based study. Neurology. 1998; 50:1694–1698.
30. Pico F, Labreuche J, Seilhean D, Duyckaerts C, Hauw JJ, Amarenco P. Association of small-vessel disease with dilatative arteriopathy of the brain: neuropathologic evidence. Stroke. 2007; 38:1197–1202.
31. Kalaria RN, Erkinjuntti T. Small vessel disease and subcortical vascular dementia. J Clin Neurol. 2006; 2:1–11.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download