INTRODUCTION
METHODS
Topographic prominence
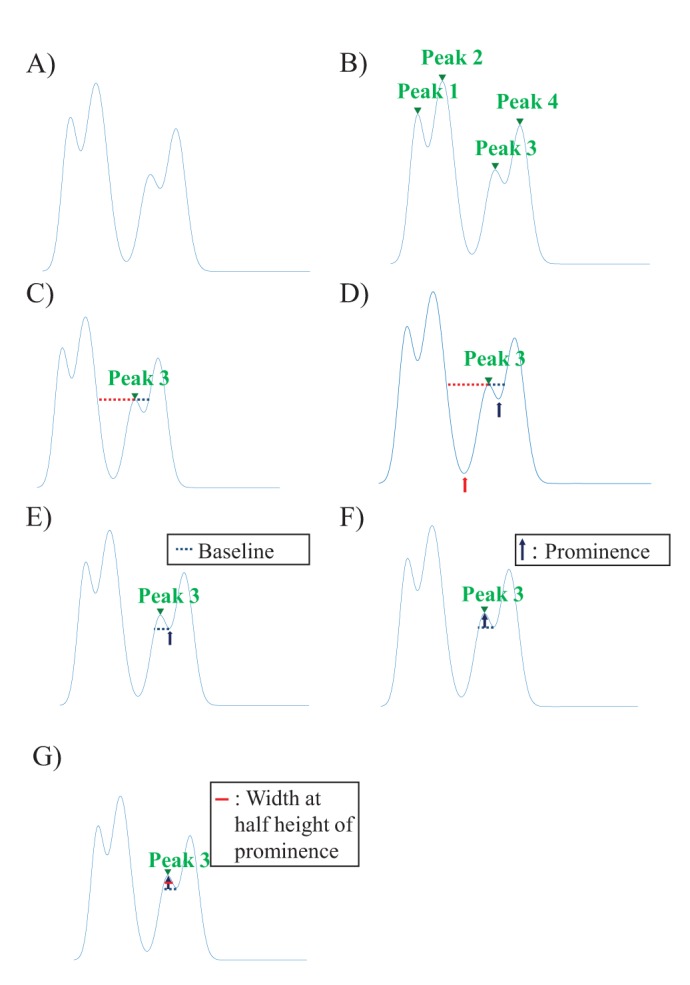 | Fig. 1Concept of the width at the half height of the prominence.(A) The raw data which could be any kind of data, for example, recorded neuronal signal, geological height information, etc. (B) The process of finding local peaks of the signal. (C) Extending horizontal lines from the peak found (peak 3, green arrow head) toward the left (red colored dotted line) and right direction (black colored dotted line). (D) Finding the minimum point of each valley below each horizontal line. (E) Selecting the baseline of peak 3. (F) Defining the height of the prominence (black arrow) of peak 3. (G) Calculating the width at the half height of the prominence.
|
Artifact subtraction
 | Fig. 2Flow chart of artifact subtraction.The depegging step changes saturated artifact value to zero. After the depegging, the remaining signals are filtered with 100 Hz high pass for baseline stabilization. In the process of TP-adopted FB filtering, residual artifacts (over 1.6 ms duration) are separately examined. If the duration at the half height of prominence is under 0.4 ms or if the full duration of the wave is under 1.6 ms, the signal is processed with 500 Hz high pass filtering for spike detection. The signal containing the spike-candidates is then thresholded for spike detection.
|
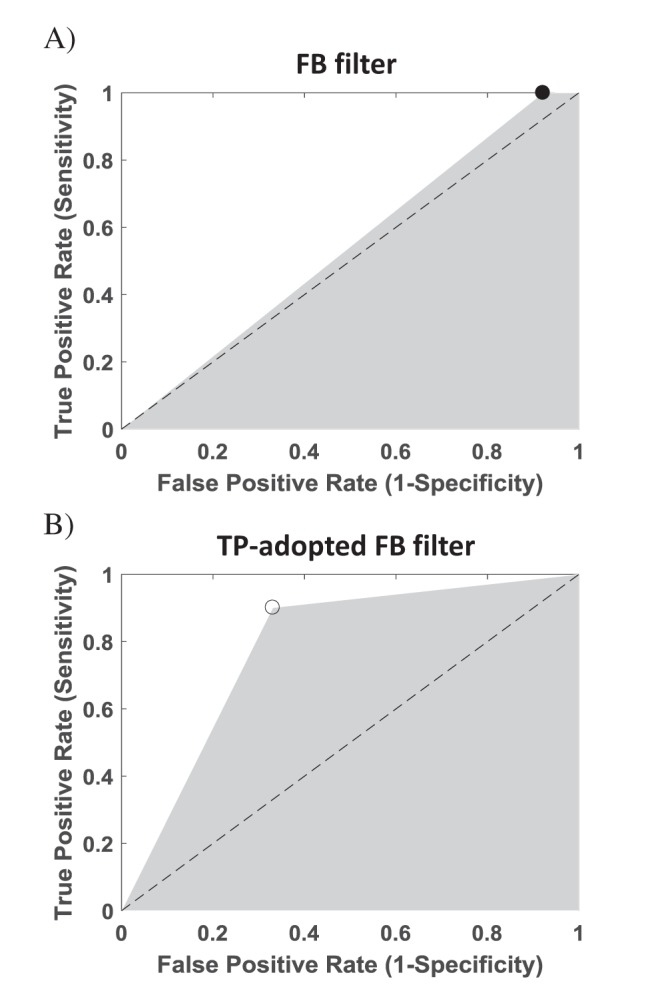
Receiver operating characteristics
Retinal preparation
Electrode and data recording system
Electrical stimulation
 | Fig. 3MEA recording and electrical stimulation.(A) One electrode was used for stimulation (asterisk in the center), while all the others for recording. The recorded signals were classified into four groups by distances between the stimulus and recording electrodes on MEA (200~400, 400~600, 600~800, 800~1000 µm). (B) The stimuli consists of cathodic phase-1st biphasic current pulses (Duration (D): 500 µs, Amplitude intensity (A): 5, 10, 20, 30, 40, 50, 60 µA, Inter-stimulus interval (I): 1000 ms, Repetition (R): 50 times).
|
RESULTS
Comparison of false positive error at 200~400 µm inter-electrode distance
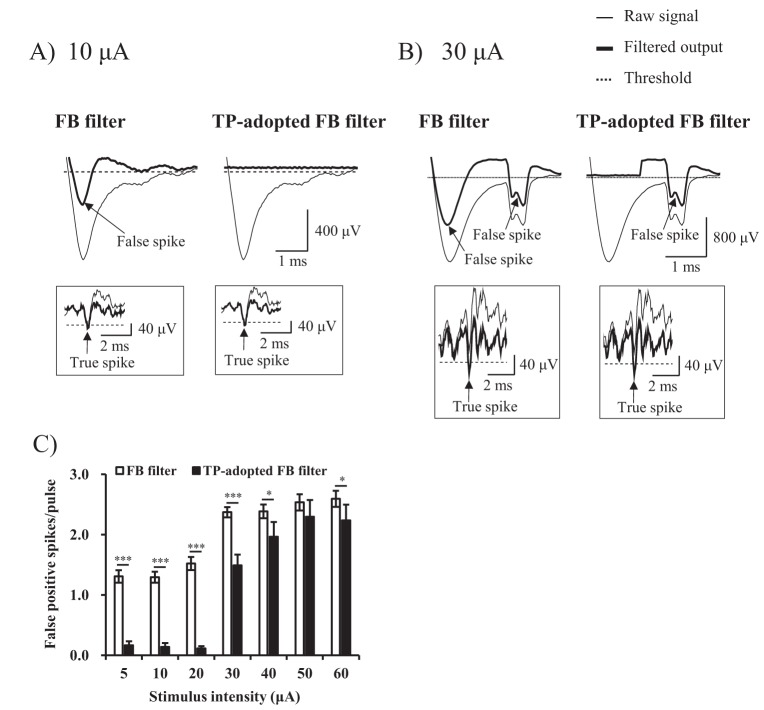 | Fig. 4Comparison of false positive error at 200~400 µm inter-electrode distance.(A, B) The performance of two algorithms at stimulus intensity of 10 µA and 30 µA were shown respectively. The thin and thick lines represent raw signal, and filtered output (artifact-subtracted) signal respectively. The dotted line represents threshold value for sorting RGC spikes from noise. The arrows indicate false positive spikes (Inset: true positive spike). (C) False positive error rates (false positive spikes/pulse) of two algorithms were statistically analyzed at all stimulus intensities.
|
Comparison of false negative error at 200~400 µm inter-electrode distance
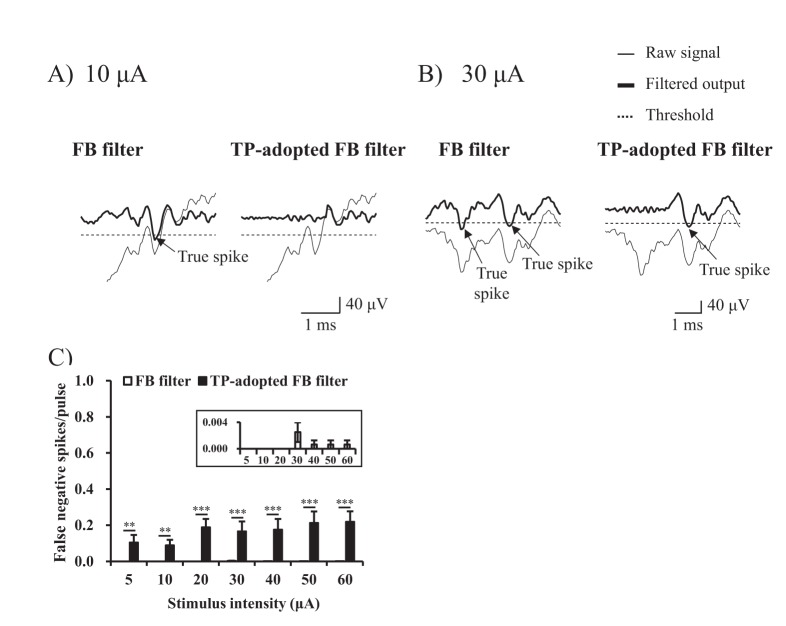 | Fig. 5Comparison of false negative error at 200~400 µm inter-electrode distance.(A, B) The performance of two algorithms at stimulus intensity of 10 µA and 30 µA were shown respectively. The thin and thick lines represent raw signal, and filtered output (artifact-subtracted) signal respectively. The dotted line represents threshold value for sorting RGC spikes from noise (Symbols: arrow=true positive spike). (C) False negative error rates (false negative spikes/pulse) of two algorithms were statistically analyzed at all stimulus intensities (Inset: To view false negative error rates of FB filter, the scale was zoomed in).
|
Comparison of false positive and false negative error according to incremental distances of electrodes
 | Fig. 6Comparison of false positive error according to incremental distances of electrodes.(A, B) The performance of two algorithms at stimulus intensity of 10 µA and 30 µA were shown respectively at 600~800 µm inter-electrode distance. The thin and thick lines represent raw signal, and filtered output (artifact-subtracted) signal respectively. The dotted line represents threshold value for sorting RGC spikes from noise. The arrows indicate false positive spikes (Inset: true positive spike). (C~E) False positive error rates (false positive spikes/pulse) of two algorithms at all stimulus intensities were statistically analyzed in terms of inter-electrode distance.
|
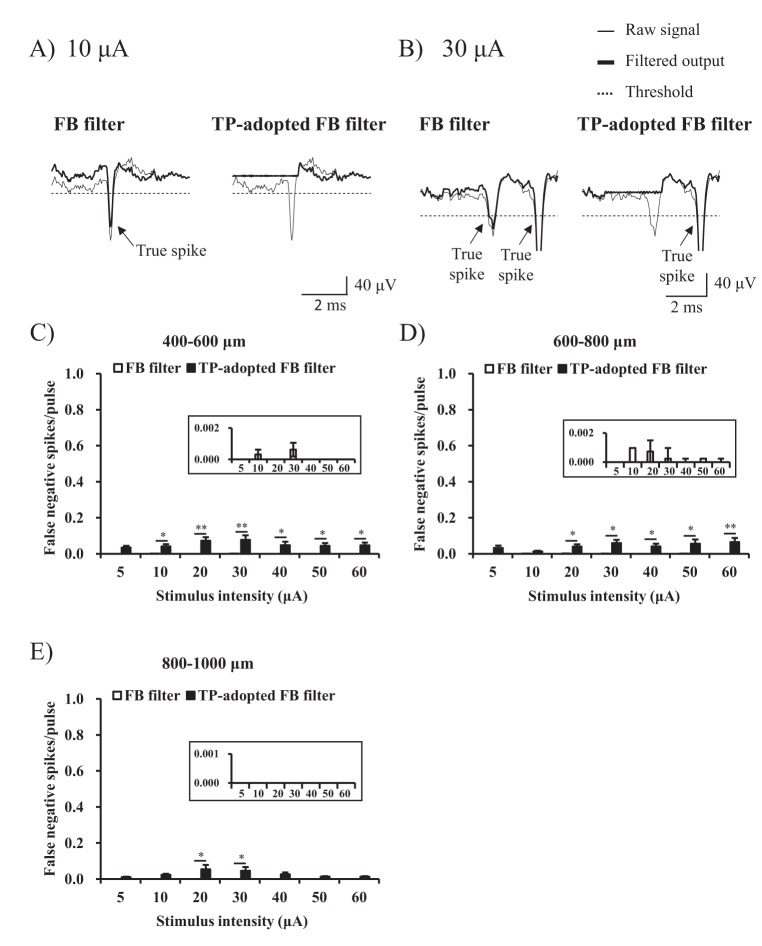 | Fig. 7Comparison of false negative error according to incremental distances of electrodes.(A, B) The performance of two algorithms at stimulus intensity of 10 µA and 30 µA were shown respectively at 600~800 µm inter-electrode distance. The thin and thick lines represent raw signal, and filtered output (artifact-subtracted) signal respectively. The dotted line represents threshold value for sorting RGC spikes from noise (Symbols: arrow=true positive spike). (C~E) False negative error rates (false negative spikes/pulse) of two algorithms at all stimulus intensities were statistically analyzed in terms of inter-electrode distance (Inset: To view false negative error rates of FB filter, the scales were zoomed in).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download