INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary intracranial tumor in adults [
12]. Because patients with GBM have a poor prognosis with a 5-year survival rate of 5% under optimal treatments, GBM is considered to be the most malignant glial tumor [
3]. Common features of GBM include invasion into surrounding normal brain tissues, accelerated proliferation, the presence of necrosis, and high degree of infiltration of diverse immune cells, including microglia, microphages, and lymphocytes [
45]. These remarkable features are believed to generate a GBM-specific tumor microenvironment (TME), which plays a crucial role in tumor progression, immune escape, local invasion, and metastasis of GBM [
678].
The tumor and its surrounding microenvironment are closely related and interact constantly [
9]. During the stages of tumor progression, a hypoxic TME or pro-oxidant-enriched TME is formed; these TMEs have both beneficial and harmful effects on tumor cells and their niche. The TME is characterized by low levels of glucose and amino acids, acidosis, and hypoxia [
1011]. A large tumor mass often produces a hypoxic TME, which is an important and common denominator of the complex metabolic changes and differentiation of cancer cells, leading to increased metastasis, invasion of cancer cells, and patient mortality [
12]. A high degree of infiltration of diverse immune and inflammatory cells into tumor tissues contributes to the development of a pro-oxidant enriched TME. Reactive oxygen species (ROS) are known to mediate cancer cell invasion and metastasis by inducing Met overexpression, Matrix metalloproteinases (MMPs) secretion, and invadopodia formation [
13]. Invasion of cancer cells into adjacent normal brain tissues and infiltration of immune cells into tumor tissues, which are important key features of GBM, are believed to be caused by interactions between the TME and cancer cells [
45].
Chemokines are important mediators of the TME that regulate invasion and metastasis of cancer cells and infiltrated immune cells in diverse malignant tumors [
1415]. Chemokines are a large group of small chemotactic proteins that guide cell migration, control cells of the immune system, and promote angiogenesis. Chemokines are classified into 4 highly conserved groups (C, CC, CXC, and CX3C) based on the position of the first two cysteines adjacent to the amino terminus [
16]. Among them, IL-8 acts as an important modulator of melanoma growth by enhancing cancer cell proliferation [
17]. In GBM, IL-8 has been implicated in glioma angiogenesis and increased malignancy [
18]. IP-10 (CXCL10) stimulation increases ERK1/2 activation, which contributes to the proliferation of GBM cells [
19]. Induction of IL-6 is known to enhance proliferation and migration of GBM cells, by activating STAT3 [
20].
In the present study, we investigated whether chemokine expression in GBM cells is affected by differential conditions of the TME, such as hypoxic and pro-oxidant-enriched micro-environments. Since the availability of glucose and acidity of the TME are known to modify the metabolic behavior of cancer cells, we also examined the effect of different concentrations of glucose on chemokine expression in either hypoxic or oxidant-enriched microenvironments.
Go to :

METHODS
Cells
Human astroglioma CRT-MG, U251-MG, and HMO6 human microglial cells were maintained in Dulbecco's modified essential medium (DMEM, WelGENE Inc., Daegu, Korea) with 10% fetal bovine serum (Gibco, Grand Island, NY 14072, USA), 1% penicillin-streptomycin in a humidified 5% CO2 incubator at 37℃.
Enzyme-linked immunosorbent assay (ELISA)
Chemokine levels were measured in the culture supernatants of CRT-MG, U251-MG, and HMO6 in 60-mm culture plates at a final concentration of 9×105 per well. After stabilization, cells were treated with either hydrogen peroxide (H2O2) or cobalt chloride (CoCl2) for 24 h. For low and high glucose conditions, cells were cultured in low glucose DMEM (1,000 mg/L glucose), and high glucose DMEM (4,500 mg/L glucose), respectively. After treatment for 24 h, supernatants were collected, and IL-6, IL-8/CXCL8, and IP-10/CXCL10 concentrations were determined using a sandwich ELISA method with human IL-6 (BioLegend, San Diego, CA, USA), IL-8 (BioLegend), and IP-10 BD OptEIA™ (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's instructions. The concentration of chemokines in each sample was determined by referencing a standard curve generated using known amounts of IL-6, IL-8, and IP-10.
RNA isolation and RT-PCR
Total cellular RNA was isolated using RNA extract kit (Easy-Blue™, Intron, Gyeonggido, Korea) according to the manufacturer's protocol. cDNA was synthesized from 5 µg of total RNA with reverse transcription and PCR was performed using 1~9 µl cDNA with a thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA). The primer sequences were as follows: GAPDH (glyceraldehyde-3-phosphate-dehydrogenase), forward 5′-GGAGCCAAAAGGGTCATCAT-3′, reverse 5′-GTGATGGCATGGACTGTGGT-3′; IL-6, forward 5′-ATGAACTCCTTCTCCACAAGC-3′, reverse 5′-GTTTTCTGCCAGTGCCTCTTTG-3′; IL-8, forward 5′-CTCCAAACCTTTCCACCCC-3′, reverse 5′-GATTCTTGGATACCACAGAGAATG-3′; IP-10, forward 5′-GTACCTCTCTCTAGAACCGTACG-3′, reverse 5′-GAGATCTTTTAGACCTTTCC-3′.
Co-culture assays of tumor cell with microglia
For indirect co-culture system, 8.0 µm-pore polycarbonate membrane transwell inserts (Corning Inc., Corning, NY, USA) were used. CRT-MG and U251-MG cells were plated on placed on the plastic surface of 24 well-plates (1×105 cells/well), then the HMO6 microglia cells were seeded onto the 8.0 mm microporous membranes of the Transwell insert (5×104 cells/well). On the following day, cells were treated with either H2O2 or CoCl2 for 24 h. For low and high glucose conditions, cells were cultured in low glucose DMEM (1,000 mg/L glucose), and high glucose DMEM (4,500 mg/L glucose), respectively.
Human phosphokinase array
Cells were treated with or without H2O2 for 24 h in either high- or low-glucose medium, and then lysed with array lysis buffer. The protein concentration was quantified using the BCA assay. Cell lysates were applied to the phosphoprotein array following the manufacturer's instructions (Proteome Profiler™ Human Phosphokinase Array kit, R&D Systems). Blocking buffer was incubated with each membrane for 1 h on a rocking platform shaker. Cell lysates were diluted in array buffer and incubated overnight at 4℃. The array was incubated in each reconstituted detection antibody cocktail for 2 h at RT, and then was diluted streptavidin-HRP for 30 min, followed by application of chemiluminescent reagent. Chemiluminescent signals were quantified with the use of the luminescent image analyzer (LAS 3000 imaging system; Fujifilm Corporation, Tokyo, Japan). The phosphokinase activity was measured by the changed spot intensity divided by reference spot intensity, using Image J software (version 1.6.0, U.S. National Institutes of Health).
Statistical analysis
The mean±standard deviation is shown in the bar graphs for the independent experiments, and statistical significance was analyzed by using ANOVA and the Student's t-test with the SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). A value of p<0.05 was regarded as statistically significant.
Go to :

DISCUSSION
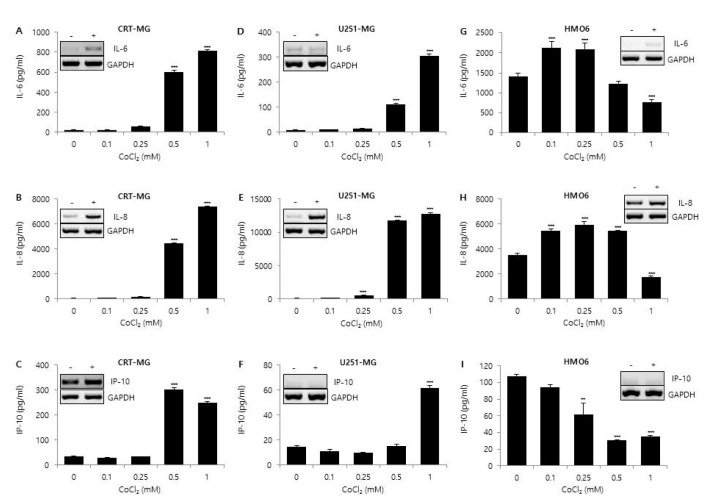
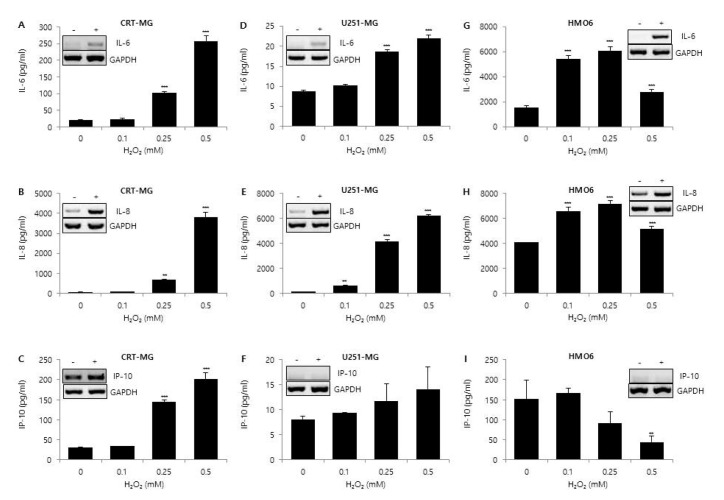
In this study, we demonstrate that hypoxia, ROS, and differential concentration of glucose influence the expression of cytokines and chemokines, such as IL-6, IL-8, and IP-10, in human glial cell lines. Treatment with CoCl2 and H2O2 significantly increased the expression levels of IL-6, IL-8, and IP-10 in a dose-dependent manner in CRT-MG and U251-MG astroglioma cells, but not in HMO6 microglia cells. Interestingly, low glucose level abrogated the stimulatory effect of H2O2 on the expression of IL-6 and IP-10 in CRT-MG cells. Our results provide evidence that astroglioma and microglia cells exhibit distinct patterns of cytokine and chemokine expression in response to CoCl2 and H2O2 treatment and that different concentrations of glucose differentially regulate the expression level of these chemokines under either hypoxic or oxidant-enriched conditions.
Treatment with CoCl2 and H2O2 significantly increased the level of chemokine expression in a dose-dependent manner in CRT-MG and U251-MG astroglioma cells. When HMO6 cells were incubated under the same conditions, we found that the levels of IL-6 and IL-8 were increased at low concentrations (0.1~0.25 mM) of CoCl2 and H2O2, but decreased at high concentrations (>0.5 mM) of CoCl2 and H2O2. Furthermore, IP-10 production in HMO6 was decreased in response to CoCl2 and H2O2 in a dose-dependent manner. To assess the cytotoxicity of CoCl2 and H2O2 using the CCK-8 assay, we found the viability of astroglioma cells and HMO6 microglia was not affected by 24 h incubation with CoCl2 or H2O2 at a concentration of up to 1 and 0.5 mM, respectively (data not shown). These results indicate that decreased expression of IL-6, IL-8, and IP-10 in microglia in response to higher concentrations of CoCl2 and H2O2 is not due to cell death.
Microglia are believed to function as major players in brain inflammation [
22]. In addition to microglia, astrocytes are also thought to actively participate in the process of neuroinflammation [
23]. Although the pathogenic mechanism of astrocytes has not been fully elucidated, it is commonly accepted that astrocytes exert a modulatory effect on brain inflammation and neurodegenerative disorders by inducing a variety of biological processes, including neuroprotection, glial scar formation and CNS inflammation [
24]. Acute inflammation in the brain is typically characterized by rapid activation of microglia; however, various cell types in the brain such as microglia, astrocytes, endothelial cells, and other glial cells produce cytokines and chemokines. These cytokines and chemokines can have both beneficial and detrimental effects in brain injury and disease, depending on the amount and duration of their release [
25]. In this study, we demonstrate for the first time the evidence that different concentrations of glucose differentially regulate the expression level of these chemokines and activation of kinases under either hypoxic or oxidant-enriched conditions. Although the precise molecular mechanisms for mechanical link between the reduced activation of kinases and the resultant decrease in H
2O
2-induced chemokine production in low glucose condition are largely unknown, our data support the notion that low glucose level influences the activation/phosphorylation of various signaling molecules, as well as chemokine expression, in astroglioma cells which actively participate in the establishment of tumor microenvironment. It is important to understand the cellular mechanisms underlying tumor microenvironment of glioblastoma for improving prognosis for patients and developing improved therapy as a therapeutic target in the future.
Astrocytes and glioblastoma cells secrete IL-8 in response to several stimuli, including LPS, IL-1β, TNF-α, ischemia, and hypoxia [
26]. In our system, treatment with CoCl
2 and H
2O
2 resulted in a significant increase in the expression of IL-8 in CRT-MG and U251-MG astroglioma cells (
Figs. 1 and
2). Moreover, compared to other cytokines, the expression level of IL-8 remained relatively similar in cells cultured with both low and high concentrations of glucose (
Figs. 3 and
4). Previously, IL-8 has been implicated in glioma angiogenesis and increased malignancy in GBM [
18]. In addition, IL-8 acts as an important modulator of melanoma growth by enhancing cancer cell proliferation [
17]. In addition to IL-8, various cytokines and chemokines have been reported to be secreted by astrocytes and GBM. IP-10 (CXCL10) induces ERK1/2 activation and contributes to the proliferation of GBM cells [
19]. Induction of IL-6 is known to enhance cell proliferation and migration in GBM cells, through STAT3 activation [
20]. In addition, CCL2 triggers migration and invasion of GBM cells and exerts paracrine effects on the microenvironment of GBM by stimulating migration of astroglial cells [
27]. Overexpression of CXCL3 and CXCL10 in GBM plays an important role in tumor growth and progression [
28]. The CXCL12-CXCR4/R7 system plays a central role in tumor development and tumor cell proliferation via an autocrine/paracrine mechanism and contributes to the dissemination and invasiveness of several human cancers, including pancreatic, colon, ovarian, prostate, breast, renal carcinomas, lymphoma, melanoma, neuroblastoma, and GBM [
29]. In this paper, we have examined the expression of these three chemokines, but it is necessary to confirm the effect of hypoxia, ROS, and differential concentration of glucose on the expression of other chemokines in the future.
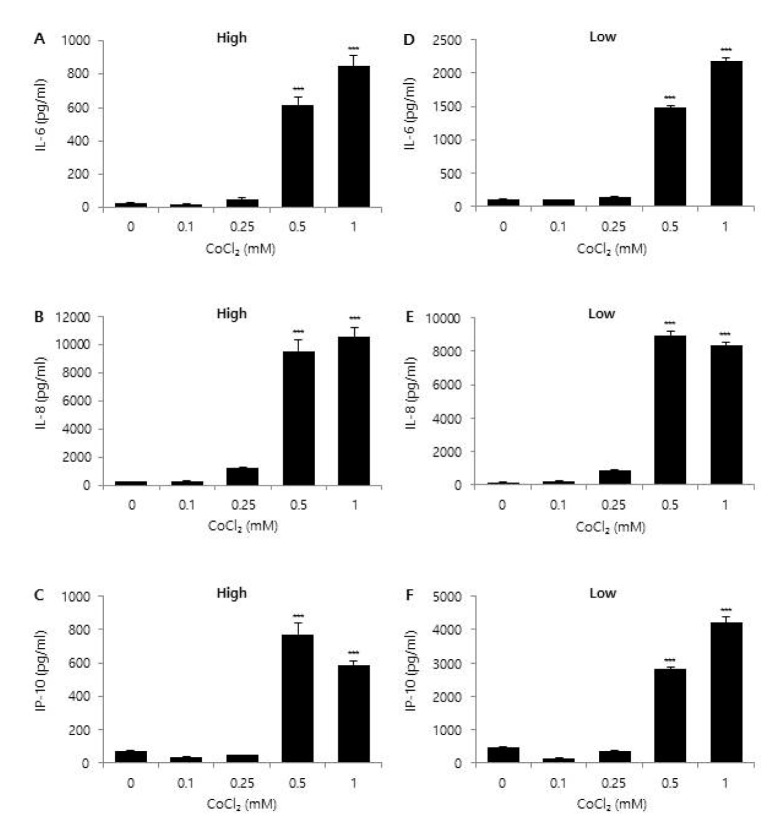
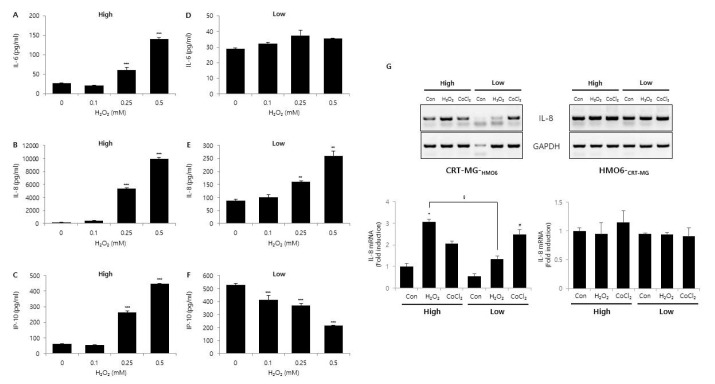
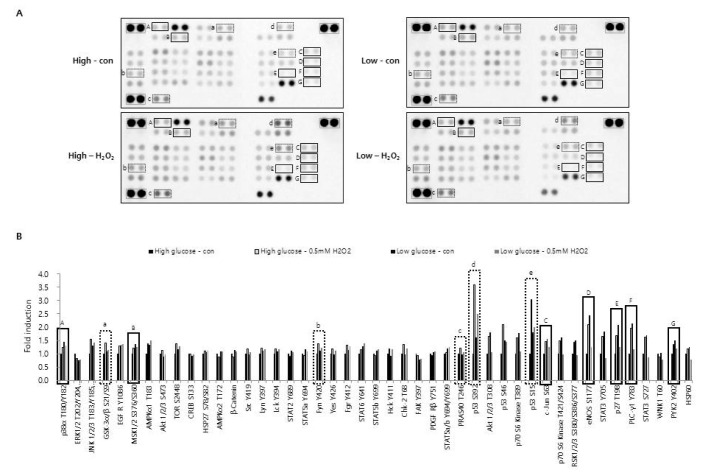
High glucose is known to affect the expression pattern of chemokines and cytokines, leading to inflammation and leukocyte activation [
21]. Moreover, an abundance of glucose is known to modify the metabolic behavior of cancer cells [
30]. These results suggest that glucose concentration may have different effects on various TMEs, such as a hypoxic or pro-oxidant-enriched microenvironment. In this study, we examined the effect of different concentrations of glucose on chemokine and cytokine expression under either hypoxic or oxidant-enriched conditions, and found that different concentrations of glucose influenced the expression patterns of IL-6, IL-8, and IP-10 following CoCl
2 and H
2O
2 exposure. As shown in
Fig. 5, low glucose condition influenced and reduced the activation/phosphorylation of various signaling molecules as well as chemokine expression in H
2O
2-treated CRT-MG cells. Under the oxidant-enriched condition, no effect of low glucose on induction of chemokine expression was observed, whereas the CoCl
2-induced hypoxic condition and low concentration of glucose showed synergistic effects on chemokine expression, at least for IL-6, IL-8, and IP-10 in CRT-MG cells (
Figs. 3 and
4). Collectively, these data suggest that low glucose level may abrogate the stimulatory effect of H
2O
2 on the expression of protumorigenic cytokines and chemokines in glioblastoma cells. The data also suggest that low glucose conditions may have different consequences when combined with other conditions in GBM.
Here, we examined the effect of CoCl2, a mimetic of hypoxia, and H2O2 on the expression of chemokines such as IL-6, IL-8, and IP-10 in CRT-MG and U251-MG astroglioma cell lines and HMO6 microglia cell line. We show that astroglioma cells and microglia exhibit distinct patterns of cytokine and chemokine expression in response to CoCl2 and H2O2 treatment, and different concentrations of glucose influence IL-6, IL-8, and IP-10 expression under either hypoxic or oxidant-enriched conditions. Interestingly, our results demonstrate that low glucose condition abrogated the stimulatory effect of H2O2 on the chemokine expression in astroglioma cells, but not in HMO6 microglia cells. These results suggest that microglia consistently secrete these chemokines in response to varied conditions of tumor microenvironment, compared to GBM cells; however, astroglioma cells could act as major players in GBM tumor microenvironment through producing more chemokines than microglia in response to some stimuli.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download