INTRODUCTION
METHODS
Animals
Stereotaxic virus injection and optic cannula implantation
Behavior
Conditioned place preference (CPP, Fig. 1)
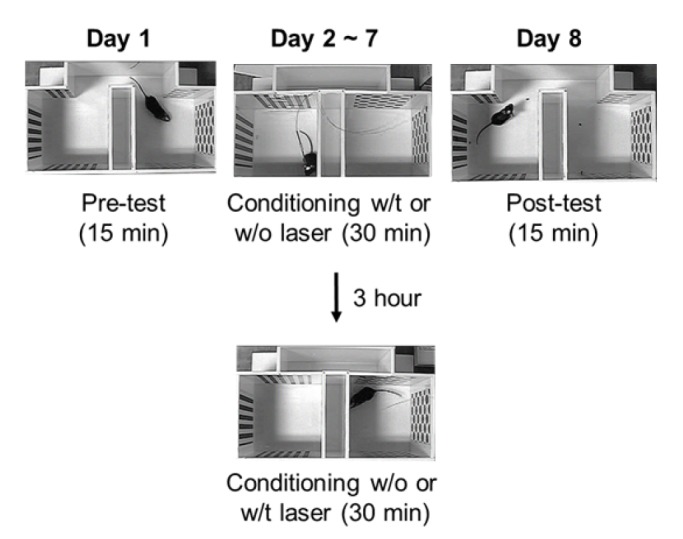 | Fig. 1Experimental schematic of the conditioned place preference testThe CFA-injected mice were first freely exposed to the apparatus for 15 min (Day 1, Pre-test). Next, the mice were conditioned with light in the compartment with less preference, and without light in the compartment with more preference (Days 2~7, conditioning). After 6 days of conditioning, the mice were assessed for changed preference for the conditioning compartments (Day 8, Post-test).
|
Electronic von Frey test
Immunohistochemistry
Data analysis
RESULTS
Inhibition of excitatory neurons in the ACC induces place preference
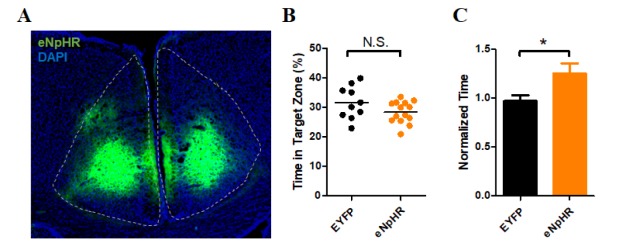 | Fig. 2Conditioned place preference in the CaMKII-eNpHR group.(A) Sample image of eNpHR expression in the ACC. (B) Result of exploration time in the less preferred compartment on Day 1 (Pre-test). There was no significant difference between the EYFP (31.62±1.74%, n=10) and eNpHR (28.39±0.98%; n=14) groups in exploration time (p=0.0978, unpaired t-test). (C) Result of the time spent in the conditioned compartment on Day 8 (Post-test, normalized to the pre-test exploration time). There was a significant difference between the EYFP (0.97±0.06, n=10) and eNpHR (1.25±0.10; n=14) groups (p=0.0441, unpaired t-test).
|
Activation of PV inhibitory neurons did not induce place preference
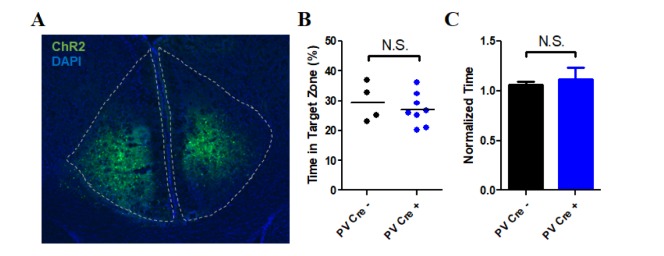 | Fig. 3Conditioned place preference in the PV-ChR2 group.(A) Sample image of ChR2 expression in the ACC. (B) Result of exploration time in the less preferred compartment on Day 1 (Pre-test). There was no significant difference between the cre(−) (29.50±3.23%, n=4) and cre(+) (27.11±1.90%, n=8) groups in exploration time (p=0.5110, unpaired t-test). (C) Result of the time spent in the conditioned compartment on Day 8 (Post-test, normalized to the pre-test exploration time). There was no significant difference between the cre(−) (1.06±0.03, n=4) and cre(+) (1.16±0.12, n=8) groups (p=0.7481, unpaired t-test).
|
Inhibition of excitatory neurons in the ACC modulates the paw withdrawal response
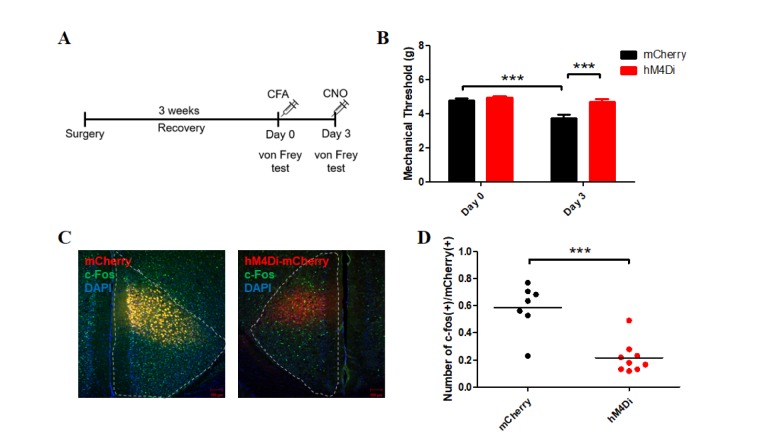 | Fig. 4Results of immunohistochemistry and electronic von Frey test in the CaMKII-hM4Di group.(A) Experimental schematic of the electronic von Frey test. Mice were allowed to recover from brain surgery for about 3 weeks. On Day 0, CFA was injected into the hind paw of each mouse, after determining the baseline response to the electronic von Frey apparatus. On Day 3, CNO was administered to each mouse 40 min before the experiment, and the response to the von Frey apparatus was measured. (B) Mechanical threshold responses to the electronic von Frey test on Days 0 and 3. There was no significant difference between the mCherry (4.81±0.09 g; n=20) and hM4Di (4.94±0.10 g; n=19) groups in the baseline responses (p=0.507, post hoc Bonferroni test) on Day 0. After CNO administration to both groups, the mechanical threshold showed a significant difference between groups (hM4Di [4.70±0.16; n=19], mCherry [3.76±0.19; n=20], p<0.001, post hoc Bonferroni test) on Day 3. The mCherry group displayed significantly different mechanical thresholds between days (Day 0 vs. Day 3, p<0.001). The mechanical threshold of the hM4Di group was not significantly different between days (Day 0 vs. Day 3, p=0.15). Repeated-measures two-way ANOVA with post hoc Bonferroni correction demonstrated a significant effect of CNO on Day 3 (p<0.001), significant effect of DREADD (p=0.002), significant difference between days (p <0.001), and a significant effect of interaction between two factors (Virus x Time, p=0.0013). (C) Images of the ACC of the mCherry (left) and hM4Di (right) groups after immunohistochemistry. (D) The ratio of activated neurons labeled by c-Fos among mCherry(+) neurons in the mCherry group was significantly different from that in the hM4Di group (p=0.0002, unpaired t-test).
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download