Abstract
Myotonia congenita (MC) is a genetic disease that displays impaired relaxation of skeletal muscle and muscle hypertrophy. This disease is mainly caused by mutations of CLCN1 that encodes human skeletal muscle chloride channel (CLC-1). CLC-1 is a voltage gated chloride channel that activates upon depolarizing potentials and play a major role in stabilization of resting membrane potentials in skeletal muscle. In this study, we report 4 unrelated Korean patients diagnosed with myotonia congenita and their clinical features. Sequence analysis of all coding regions of the patients was performed and mutation, R47W and A298T, was commonly identified. The patients commonly displayed transient muscle weakness and only one patient was diagnosed with autosomal dominant type of myotonia congenita. To investigate the pathological role of the mutation, electrophysiological analysis was also performed in HEK 293 cells transiently expressing homo- or heterodimeric mutant channels. The mutant channels displayed reduced chloride current density and altered channel gating. However, the effect of A298T on channel gating was reduced with the presence of R47W in the same allele. This analysis suggests that impaired CLC-1 channel function can cause myotonia congenita and that R47W has a protective effect on A298T in relation to channel gating. Our results provide clinical features of Korean myotonia congenita patients who have the heterozygous mutation and reveal underlying pathophyological consequences of the mutants by taking electrophysiological approach.
Myotonia congenita is an inherited disease clinically characterized by episodic generalized stiffness of skeletal muscle upon sudden forceful movements [12]. According to the inheritance patterns, myotonia congenita is divided into autosomal dominant “Thomsen disease”, and autosomal recessive “Becker disease” [123]. The clinical features of myotonia congenita display impaired muscle relaxation, transient or permanent muscle weakness, muscle hypertrophy, muscle pain, and cold sensitivity with a little variance for each patient [4]. This condition can be caused by mutations in CLCN1, located on chromosome 7q35, consisting of 35kb DNA and 23 exons [156]. CLCN1 codes for CLC-1 channel majorly expressed in skeletal muscle and contributing 80~90% of resting membrane conductance (Gm), thereby responsible for determining muscle excitability [7].
The CLC-1 channel belongs to a member of a large family comprising Cl− channels and Cl−/H+ exchangers [891011]. The structure of CLC-1 channel still remains unveiled, but it is confirmed that the CLC family proteins including channel and exchanger is formed by a homodimeric structure with separate pores and independent transport pathway [1213]. Each subunit consists of highly conserved 18 transmembrane domains as well as two cystathionine beta synthase (CBS) domains in its carbonyl end [1213]. The gating of the CLC-1 is regulated by two processes called fast gating (protopore) and slow gating (common) [14]. The fast gate closes and opens each subunit independently with fast kinetics in less than 1 ms at positive potentials and the slow gating (common) enables both subunits to gate simultaneously with slow kinetics [14]. The CLC-1 channel is activated by relatively higher voltage than resting membrane potential caused by depolarization and stabilizes the resting membrane potential of skeletal muscle by influx of chloride ions [1015]. Impaired function of CLC-1 enables potassium ions expelled in repolarization phase to be accumulated in extracellular transverse tubular systems, causing the elevation of resting membrane potential according to Nernst equation [10]. Thus, involuntary contractions of skeletal muscles result in muscle stiffness.
Previously, we have screened 23 exons of CLCN1 in 14 unrelated Korean patients with myotonia congenita and a part of the results were published elsewhere [16]. Among them, we have identified four patients having two identical mutations on CLCN1, R47W and A298T. In this study, we would like to present clinical features of these patients and performed an electrophysiological analysis to understand pathological background of these CLC-1 mutants.
Patients with R47W (c.139C>T) and A298T (c.892G>A) on CLCN1 consisted of three males and one female. The study was reviewed and approved by Pusan National University Hospital Institutional Review Board. Informed consents for DNA acquisition and analysis were provided in written form from all patients. Clinical features of the patients are listed on Table 1.
Direct sequence analysis of entire coding regions of CLCN1 was performed to identify the mutations in CLCN1. DNA was obtained from anticoagulated whole blood of each patients. Sequence specific primer pairs which cover whole coding regions of CLCN1 was used for PCR [5]. For patients who underwent muscle biopsy, biopsied skeletal muscle was also used for reverse transcription-polymerase chain reaction (RT-PCR)-based sequence analysis. Total RNA was extracted from frozen muscle tissue and reverse transcribed to yield 1 µg of total cDNA. The primary cDNA strand was amplified through PCR. Six overlapping cDNA primer pairs which were presently designed were used for amplification of cDNA. DNAs that were amplified by PCR were separated on 2% agarose gel, purified, cycle-sequenced with PCR primers using the BigDye™ Terminator Sequencing Kit (Applied Biosystems, Foster, CA, U.S.A.), and electrophoresed using an ABI PRISM® 3730XL DNA analyzer (Applied Biosystems).
To assess the allele frequency of R47W (c.139C>T) and A298T (c.892G>A) among normal Koreans, PCR-restriction fragment length polymorphism (RFLP) analysis was performed in 100 normal Korean controls using restriction enzymes BspE1 (for c.139C>T) and CspC1 (for c.892G>A), respectively. For c.139C>T (R47W), an addition of restriction enzyme BspE1 loses the normal recognition site, and produce single DNA fragment of 454 bp, while WT DNA breaks into two fragments (343 bp+112 bp). Likewise, c.892G>A (A298T) mutation produces single DNA fragment of 279 bp, while WT breaks into three fragments (98 bp+35 bp+146 bp) upon addition of CspC1. Primer sequence used for PCR-RFLP analysis is available upon request.
Because some patients with typical myotonia congenita may also be caused by SCN4A mutations, we also screened SCN4A using direct DNA sequencing as described before [17].
All patients were asked for detailed clinical histories, and were examined by neuromuscular clinical experts. Clinical history parameters included age of onset, distribution of muscle stiffness, the presence of transient or permanent muscle weakness, provoking factors, and family history. Physical examinations were focused on the presence of muscle hypertrophy, individual muscle power, and types of maneuvers provoking myotonia. Needle electromyography (EMG) was performed on at least two different muscles in each extremity. Routine laboratory tests included complete blood count; liver, renal and thyroid function tests; blood glucose and electrolytes; serum creatine kinase (CK) level; chest radiography; and electrocardiogram. Biopsy of skeletal muscle was performed in two patients on biceps brachii muscles. Biopsied muscles were rapidly frozen in isopentane solution which was pre-chilled using liquid nitrogen, and were processed for tissue histochemical staining reactions including hematoxylin-eosin, modified Gomori trichrome, NADH-tetrazolium reducatse, periodic acid-Schiff (PAS), cytochrome oxidase (COX), and ATPase.
Human CLCN1 DNA in the pRc/CMV vector was subcloned into the pEGFP-N1 vector (Clontech) using the enzyme site Xho1 and EcoR1. The mutants were introduced by employing the QuickChange site-directed mutagenesis kit (Stratagene). hCLCN1 gene was provided in kind by Dr. Alfred. L. George of Vanderbilt University, Nashville, TN. U.S.A.
HEK293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin and incubated at 37℃ in a humidified incubator with 95% air and 5% CO2. Transient transfection was performed by using Fugene 6 (Promega).
Homology model of hCLC-1 was constructed using proved crystal structure of cmCLC (PDB id: 3ORG) which has 97% of query cover and 31% identical residues and stCLC (PDB id: 1KPL) with 87% of query cover and 25% identical residues compared to the transmembrane domain of the hCLC-1. Crystal structure of the C-terminal domain of CLC-K (PDB: 5TQQ) was also used, which shares 44% identical residues and 65% query cover compared to hCLC-1. MODELLER was used to construct the CLC-1 model and all the figures were prepared using Pymol 1.8.0.5 [1819]. N-terminal domain of the model was omitted due to lack of similar crystal structure in the N terminus.
Human CLCN1 was inserted in vector pEGFP-N1 and was transiently expressed in HEK293 cells using fugene6 transfection reagent. Patch clamp experiments in the whole cell configuration were conducted in the room temperature. Axopatch 200B amplifier and Digidata1440 with software Clampex were used for current recording. Extracellular solution (in mM) was composed of: 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES, adjusted with NaOH to pH 7.4. Intracellular solution (in mM) was composed of: 130 NaCl, 2 MgCl2, 5 EGTA, 10 HEPES, adjusted to pH 7.4 with NaOH. All values for ion concentration of extracellular or intracellular solution are indicated in mM. Relative open probabilities were fitted with a Boltzmann distribution with some modifications.
where P−165 and P+75 are the open probabilities of the channel at the most negative potential (−165 mV) and most positive potential (+75 mV or +195 mV) respectively, V is the membrane potential, V1/2 is the half-maximal activation potential, and κ is the slope factor.
R47W and A298T was identified in CLCN1 in four patients (Fig. 1). Both of them were heterozygous, but we were not able to confirm whether they are compound heterozygote or lies on the same allele. In PCR-RFLP analysis using 100 normal Korean controls, same change as a heterozygote form was observed in one for R47W and another one for A298T, respectively.
None of the patients carried SCN4A mutations and the possibility of SCN4A-related myotonia congenita was excluded.
Patient 1: The patient 1 is a 21-year-old man who complained of episodic stiffness of extremities and trunk when he attempts to start sudden exercise. He reported that his symptoms began at age 12 and none of his family members had the same symptoms. He denied episodic muscle weakness or cold sensitivity. His muscle strength was normal and no muscle hypertrophy was observed. Laboratory tests were unremarkable except for the mildly elevated serum CK levels (463 IU). Needle EMG showed myotonic discharges. Mexiletine were prescribed and the symptoms were moderately alleviated.
Patient 2: This 20-year-old man first recognized trouble in exercising at high school, when he was 16 years old, and since then exercising was accompanied by discomfort all along. There was no family history of the same symptoms he suffered. He denied a transient muscle weakness, but stated that his symptom tends to get worse when exposed to the cold temperature. Muscle hypertrophy or muscle weakness was not observed, and needle EMG showed myotonic discharges.
Patient 3: A 22-year-old man suffering intermittent muscle stiffness for two years visited our neuromuscular clinic for evaluation of his symptom. He had no affected family member, and denied episodic muscle weakness or cold sensitivity. Serum CK level was slightly elevated (203 IU), and needle EMG showed myotonic discharges in his muscles. Muscle strength was normal and muscle hypertrophy was not observed. Muscle biopsy did not show any specific abnormality. Although mexiletine and carbamazepine were tried to alleviate his symptom, the response was poor and medications were discontinued.
Patient 4: The patient 4 was a 42-year-old lady who complained occasional muscle stiffness following sudden exercise since 20 years old. Her family history was compatible with autosomal dominant inheritance. She had a mild hypertrophy in her thigh and calf muscles. Serum CK level was normal (111 IU), and needle EMG showed myotonic discharges. Muscle biopsy did not show any significant abnormality. Carbamazepine showed a dramatic alleviation of her symptom.
Since all patients have heterozygous mutation, R47W and A298T mutants were expressed as heteromeric mutants that link R47W and A298T (R47W+A298T). Homodimeric double mutant (R47W/A298T) channel and heteromeric channels that consist of WT and double mutant were also tested. To understand functional characteristics, WT CLC-1 and the mutant channels were expressed in HEK 293 cells and measured by whole-cell patch clamp recordings. The voltage steps were applied from −165 mV to +75 mV or +195 mV in 20 mV increments (Fig. 2A). The maximum chloride current density for WT and the mutants were indicated to compare the channel function (Fig. 2B). WT hCLC-1 channel produced its characteristic pattern of currents, generating inward rectification with a rapid deactivation upon hyperpolarization (Fig. 2C). Except homodimeric A298T channel that did not display the characteristic pattern of current crossover phenomenon, other mutant channels induced similar kinetics compared to WT CLC-1 (Fig. 2C). It is notable that this phenomenon disappeared when A298T is expressed with WT or R47W, suggesting that WT or R47W subunit can compensate for the channel function. The maximum chloride current densities of the mutant channels were all decreased compared to WT CLC-1 channels (Fig. 2B, C, Table 2). Among the mutant channels, homodimeric double mutant (R47W/A298T) showed significantly reduced current density (101.4±16.8 pA/pF, n=4) relative to WT CLC-1 (499.0±86.3 pA/pF, n=10) (Fig. 2B, Table 2). This result explains that the mutation found in the patients affect to generate insufficient chloride conductance. Especially, homodimeric double mutation significantly reduced current density to impede normal channel function, thereby suggesting that this mutation is plausible genotype found in the MC patients.
The gating of the mutant channels exhibited altered compared to WT. Homodimeric mutant channels, R47W and A298T led to right-shifted V1/2 at −20.0±2.8 mV and 39.4±14.9 mV, respectively (Table 2, Fig. 3A). The slope factor of channel activation in homodimeric A298T showed two-folded increase (42.5±6.9 mV) compared to that of WT (27.2±2.9 mV) (Table 2), suggesting that this mutant channel is less voltage sensitive. In contrast, V1/2 of R47W, A298T, and double mutatnt, R47W/A298T, expressed with WT all left-shifted compared to the homodimeric mutant channels (Fig. 3A). R47W and the double mutant expressed with WT produced left-shifted V1/2 to more hyperpolarizing potentials by >30 mV and >20 mV, respectively (Table 2). In contrast, the gating of A298T expressed with WT showed right-shifted V1/2 compared to WT by <20 mV, but to a lesser degree than homodimeric A298T (Fig. 3A, Table 2). This results support the idea that co-expressed WT subunit can neutralize the modified channel gating of the mutant channels by shifting V1/2 to more hyper polarizing potentials.
V1/2 of R47W and A298T co-expressed mutant (R47W+A298T) was occurred at −16.6±1.9 mV, shifting to more depolarizing potentials by >40 mV compared to WT CLC-1 channel (Table 2, Fig. 3C). The analysis of gating in R47W and A298T co-expressed channel verified that this form of mutation can lead to not only modified channel activation but also decreased current density of outwardly rectification (Table 2).
Taken together, the homodimeric mutants affect abnormal function of channel gating, but can be compensated by the expression of WT CLC-1. It is interesting that shifted V1/2 to depolarizing potentials of A298T can be moved to backward by presence of R47W.
Among 14 unrelated Korean patients with myotonia congenita (MC), four shared following two mutations in CLCN1 - c.139C>T (R47W) located in exon 1 and c.892G>A (A298T) in exon 8. Our observation that identical mutations were commonly found in significant numbers of unrelated patients strongly suggests that these are the most common CLCN1 mutations causing MC among Koreans. PCR-RFLP analysis using normal controls further suggested these two mutations are circulating among Koreans with a relatively high allele frequency (~0.5%). A298T mutation in CLC-1 was previously reported as an autosomal dominant mutation in Chinese kindred analysis [20]. The location of A298T mutation identified by homology modeling of hCLC-1 is near the dimer interface on the loop between helix H and I (Fig. 4A, 4B). This region was found a hotspot for dominant negative mutations in UK population [21], and to be involved in common gating [2223]. Functional studies of A298T mutant channel has not been performed. R47W mutation in CLCN1 has been reported to cause autosomal recessive form of the disease in Chinese population [6]. It is located in the N-terminal of CLC-1 (Fig. 4B).
To ascertain the role of each mutation, the patch clamp test for single mutant was conducted. Homozygous and heterozygous A298T displayed significant modification of channel gating with depolarizing shift in relative open probability curve and reduced average current density. When an alanine at position 298 is changed to threonine, it interrupts the relay of conformational change in alpha helices around pore region between the monomers and hinder opening of the channel, hence explaining the large depolarizing shift in relative opening probability of homozygous A298T. This can reduce chloride ion conductance in physiological settings and increase the excitability of sarcolemma therefore leading to symptoms of MC. R47W also resulted in decreased current density, but its channel activation was similar to WT when it was in the form of heterozygote (Table 2).
It is also notable that the clinical features of these four patients were relatively milder than other patients with MC. This included absence of transient or fixed muscle weakness, less frequent generalized muscle stiffness and muscular hypertrophy (Table 1). It is critical issue to determine whether the mutations R47W and A298T lie on the same allele or on each allele as a compound heterozygous form in the patients. Unfortunately, the genotype of family members for the patients were not available on this case. Furthermore, the RT/PCR analysis from patient muscle biopsies did not produce a cDNA template long enough (753 bp) since these two mutations are far apart. To ascertain these peculiar clinical and genotype features, electrophysiological analysis was conduct to characterize the hCLC-1 channels harboring various mutant combinations of WT-R47W, WT-A298T, WT-R47W/A298T, R47W-A298T.
Co-expression of R47W and A298T was conducted in three forms (homozygous, heterozygous with one normal allele, and compound heterozygous) to analyze the joint effect of the two mutations on CLC-1 function. To explain the mild symptoms of MC in patients with recurrent mutation, we hypothesized that R47W has a protective effect from the dominant negative effect of A298T, similar to a previous report [6]. WT+R47W/A298T did not show the tendency to open at more depolarizing potentials as WT+A298T did. Instead, the mutant channel gating was shifted to hyperpolarizing potentials. The chloride current may have decreased in both mutants, but WT+R47W/A298T decreased to a lesser extent. R47W/A298T exhibited normal channel gating, a marked distinction from A298T. However, chloride current density was reduced significantly which may be due to facilitated degradation of mutant proteins [24]. It seems that after dimerization to form CLC-1, mutant CLC-1 subunit with R47W and A298T less disturb the function of the other subunit than the CLC-1 with A298T alone and results in normal channel gating. Hence we suggest that within the same subunit of CLC-1, R47W mutation neutralizes pathogenic effect of A298T. Additionally, R47W+A298T exhibited further impaired gating and decreased current density than the mutations with WT (WT+R47W, WT+A298T, WT+R47W/A298T). This suggests that the absence of normal CLC-1 subunit aggravates functional impairment of the mutant channels.
Among various mutations tested and analyzed above, the possible allele combinations are the compound heterozygote R47W plus A298T on separate alleles or the double mutant allele R47W/A298T plus WT. For the latter mutant, it is not likely to be relevant to the clinical phenotype in these patients because the channel gating was not significantly modified and the current reduction displayed minor effect (25% decrease). Thus, it is more plausible that the clinical phenotype in these four patients is caused by the compound heterozygotes.
Here we first report the R47W and A298T mutation in Korean MC patients together with the combined effect of two mutations on CLC-1 function. The functional consequences of the mutants and its underlying pathology are analyzed based on the electrophysiological findings. We showed that R47W has a protective effect on channel function opposed to the dominant negative effect of A298T, especially on channel gating. Our study gives insight into the combination of mutations leading to unexpected change in channel function and varying severity of the disease. These findings can help clinicians in genetic counseling and diagnosis of MC patients.
Figures and Tables
 | Fig. 1Recurrent mutation, c139C>T and c.892G>A, of hCLCN1 found in myotonia congenita patients.(A) C to C/T at position c.139, results in a substitution of amino acid tryptophan (TGG) for arginine (CGG) at position p.47 (p.Arg47Trp). (B) G to G/A at position c.892, results in a substitution of amino acid threonin (ACT) for alanine (GCT) at position p.298 (p.Ala298Thr).
|
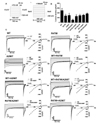 | Fig. 2The representative chloride currents recorded from HEK293 cells transiently expressing hCLC-1 WT channels and the myotonic mutants.(A) Voltage pulse protocol applied to the whole cell configuration. The voltage pulses were applied from −165 mV to +75 mV or +195 mV in 20 mV increments. A tail pulse was given at −125 mV during 20 ms and holding potential was at 0 mV. (B) The chloride current density. The maximal inwardly rectifying currents (pA) were obtained and divided by capacitance (pF). The values were recorded in absolute value. Data are means±s.e.m. n=10 (WT CLC-1), n=4 (R47W), n=4 (A298T), n=4 (WT+R47W), n=5 (WT+A298T), n=4 (WT+R47W/A298T), n=4 (R47W/A298T), n=5 (R47W+A298T). Statistical significance (*p<0.05, **p<0.01) was determined by student's t-test. (C) The kinetics and I (current)-V (voltage) relationship of heteromeric and homodimeric R47W. (Left) Current traces of WT hCLC-1 channel and the heteromeric or homodimeric mutants obtained by whole cell patch clamp. (Right) Comparison of IV relationship in WT and the heteromeric or homodimeric mutants. The maximal inwardly rectifying currents (pA) were divided by the whole cell capacitance (pF). Asterisk indicates the mutant recorded by the voltage pulse protocol to +195 mV. Closed and open square indicates WT hCLC-1 and the mutants, respectively.
|
 | Fig. 3Voltage dependence of the relative open probability obtained from WT hCLC-1 and the myotonic mutants.(A) The analysis of voltage dependence of the WT hCLC-1, homo-, or heterodimeric mutants. (Left) Illustration of tail currents from 150 ms to 170 ms. (Right) The comparison of open probability (Po) of the WT hCLC-1, homo-, or heterodimeric mutants. The peak currents at tail pulse were divided by the minimal current, and fitted to Boltzmann distributions. Black and red line indicate the WT hCLC-1, homo-, or heterodimeric mutants, respectively. (B) The comparison of voltage dependences obtained from WT hCLC-1 and the mutants. Data are means±s.e.m. n=10 (WT CLC-1), n=4 (R47W), n=4 (A298T), n=4 (WT+R47W), n=5 (WT + A298T), n=4 (WT+R47W/A298T), n=4 (R47W/A298T), n=5 (R47W+A298T).
|
 | Fig. 4Location of the mutation in homology modeling of hCLC-1 based on the crystal structure of cmCLC and CLC-K.(A) Side (left) and top (right) view of homology modeling describing the homodimeric structure of hCLC-1 channel. The structure was built based on the structure of stCLC (PDB id: 1KPL) and CLC-K (PDB id: 5TQQ). Each monomer was colored in magenta and yellow and the location of mutant corresponding to A298T was indicated in the spacefilled model in cyan. The illustrations in the box depict the position of the A298T mutant localized on the loop between helix H and I. (B) Location of the mutants, R47W and A298T depicted on the topology model of hCLC-1. The model of hCLC-1 channel was described based on the structure from Dutzler et al. [13].
|
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT (Information & Communication Technology) and Future Planning (MSIP) of the Korea government with grant number 2015R1A2A1A05001756 to I.S. This study was also supported by the Education and Research Encouragement Fund of Seoul National University Hospital (I. So).
Notes
Author contributions H.J.C., C.H.K., and K.H. performed molecular biology and electrophysiology experiments, analyzed data; H.J.C., C.H.K., D.S.K., and K.H. wrote the manuscript; J.H.S. and D.S.K. evaluated Korean myotonia congenita patients; K.H., D.S.K. and I.S. designed research; K.H. constructed homology modeling; I.S. advised on experimental design.
References
1. Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992; 257:797–800.
2. George AL Jr, Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993; 3:305–310.
3. Wu FF, Ryan A, Devaney J, Warnstedt M, Korade-Mirnics Z, Poser B, Escriva MJ, Pegoraro E, Yee AS, Felice KJ, Giuliani MJ, Mayer RF, Mongini T, Palmucci L, Marino M, Rüdel R, Hoffman EP, Fahlke C. Novel CLCN1 mutations with unique clinical and electrophysiological consequences. Brain. 2002; 125:2392–2407.
4. Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002; 82:503–568.
5. Lorenz C, Meyer-Kleine C, Steinmeyer K, Koch MC, Jentsch TJ. Genomic organization of the human muscle chloride channel CIC-1 and analysis of novel mutations leading to Becker-type myotonia. Hum Mol Genet. 1994; 3:941–946.
6. Liu XL, Huang XJ, Shen JY, Zhou HY, Luan XH, Wang T, Chen SD, Wang Y, Tang HD, Cao L. Myotonia congenita: novel mutations in CLCN1 gene. Channels (Austin). 2015; 9:292–298.
7. Pedersen TH, Riisager A, de Paoli FV, Chen TY, Nielsen OB. Role of physiological ClC-1 Cl- ion channel regulation for the excitability and function of working skeletal muscle. J Gen Physiol. 2016; 147:291–308.
8. Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991; 354:301–304.
9. Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006; 440:484–489.
10. Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2008; 43:3–36.
11. Feng L, Campbell EB, Hsiung Y, MacKinnon R. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010; 330:635–641.
12. Park E, Campbell EB, MacKinnon R. Structure of a CLC chloride ion channel by cryo-electron microscopy. Nature. 2017; 541:500–505.
13. Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002; 415:287–294.
14. Accardi A, Pusch M. Fast and slow gating relaxations in the muscle chloride channel CLC-1. J Gen Physiol. 2000; 116:433–444.
15. Fahlke C. Ion permeation and selectivity in ClC-type chloride channels. Am J Physiol Renal Physiol. 2001; 280:F748–F757.
16. Moon IS, Kim HS, Shin JH, Park YE, Park KH, Shin YB, Bae JS, Choi YC, Kim DS. Novel CLCN1 mutations and clinical features of Korean patients with myotonia congenita. J Korean Med Sci. 2009; 24:1038–1044.
17. Lee SC, Kim HS, Park YE, Choi YC, Park KH, Kim DS. Clinical diversity of SCN4A-mutation-associated skeletal muscle sodium channelopathy. J Clin Neurol. 2009; 5:186–191.
18. Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993; 234:779–815.
19. DeLano WL. Unraveling hot spots in binding interfaces: progress and challenges. Curr Opin Struct Biol. 2002; 12:14–20.
20. Gao F, Ma FC, Yuan ZF, Yang CW, Li HF, Xia ZZ, Shui QX, Jiang KW. Novel chloride channel gene mutations in two unrelated Chinese families with myotonia congenita. Neurol India. 2010; 58:743–746.
21. Fialho D, Schorge S, Pucovska U, Davies NP, Labrum R, Haworth A, Stanley E, Sud R, Wakeling W, Davis MB, Kullmann DM, Hanna MG. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain. 2007; 130:3265–3274.
22. Cederholm JM, Rychkov GY, Bagley CJ, Bretag AH. Inter-subunit communication and fast gate integrity are important for common gating in hClC-1. Int J Biochem Cell Biol. 2010; 42:1182–1188.
23. Duffield M, Rychkov G, Bretag A, Roberts M. Involvement of helices at the dimer interface in ClC-1 common gating. J Gen Physiol. 2003; 121:149–161.
24. Lee TT, Zhang XD, Chuang CC, Chen JJ, Chen YA, Chen SC, Chen TY, Tang CY. Myotonia congenita mutation enhances the degradation of human CLC-1 chloride channels. PLoS One. 2013; 8:e55930.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download