Abstract
MDL-12330A is a widely used adenylyl cyclase (AC) inhibitor that blocks AC/cAMP signaling. In this study, we demonstrated a novel antitumor activity of this drug in gastric carcinoma (GC) cell lines. In these GC cells, MDL-12330A reduced cell viability and induced cell death in a concentration-dependent manner. At a moderate concentration (~20 µM), MDL-12330A mainly induced apoptotic death whereas at concentrations greater than 20 µM, it increased non-apoptotic cell death. The induction of apoptosis was at least partially regulated by CHOP-mediated DR5 upregulation, as detected by immunoblotting and gene interference assays. More importantly, low concentrations of MDL-12330A effectively enhanced recombinant human tumor necrosis factor (TNF)-related apoptosis-inducing ligand (rhTRAIL)-induced apoptosis and clonogenicity in these gastric cancer cells. This study demonstrates a possible role of MDL-12330A as a potential sensitizer to TRAIL, and suggests a novel therapeutic strategy targeting gastric cancer cells.
Gastric cancer (GC) is one of the major causes of cancer-related death around the world [1]. Although surgery is effective treatment in early GC, it has limitations in treating advanced cases [23]. Thus, chemotherapeutic treatment is still considered to be an important option, and great efforts are being devoted to improving therapeutic strategies to treat gastric cancer.
Because it has been shown to selectively kill transformed cells while sparing normal cells, TNF-related apoptosis-inducing ligand (TRAIL) has emerged as an attractive cancer therapeutic agent [4]. Binding of TRAIL to TRAIL receptors on the cell surface initiates apoptotic signaling by recruiting adaptor proteins and initiator caspases to the death domains in the cytoplasmic tails of TRAIL receptors [5]. To date, five TRAIL receptors have been identified in human cells, and among them, TRAIL-R1 (DR4) and TRAIL-R2 (DR5) possess the death domain transducing TRAIL-mediated death signals. It is believed that the tumor selectivity of TRAIL is due to the wide distribution of DR4 or DR5 on tumor cells as well as the high expression of antagonist decoy receptors, which do not have an active death domain, on normal cells [6]. However, recent studies have shown that many cancer cells are resistant to TRAIL [7]. Hence, various strategies to overcome the TRAIL resistance of cancer cells are currently being tested. Among these strategies, the role of various sensitizers, such as natural compounds and synthetic small molecules, in combination with TRAIL are under active investigation with the aim of restoring TRAIL sensitivity [8910].
The compound N-(cis-2-phenyl-cyclopentyl)azacyclotridecan-2-imine-hydrochloride [MDL-12330A], is one of the most widely used adenylyl cyclase (AC) inhibitors, use of which decreases the concentration of cellular cAMP [11]. However, MDL-12330A has non-specific effects including enhancing the intracellular calcium concentration via inhibition of Kv channels in pancreatic beta cells [12] and affecting glycine transport in retinal Muller cells [13]. In this study, we report another important AC-independent effect of MDL-12330A. Specifically, we observed that MDL-12330A could trigger cytotoxicity, and was able to synergize with TRAIL to induce apoptosis in GCs. Here, we have investigated this novel effect of MDL-12330A, and suggest a possible role for it a TRAIL-sensitizer for future clinical development.
The SNU601 and SNU638 human GC cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea), and AGS human GC cell lines, IEC-6 and IEC-18 rat gut epithelial cell lines, and human BEAS-2B lung epithelial cell line were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in the RPMI 1640 medium (Invitrogen, CA) supplemented with 10% (v/v) fetal bovine serum and 1% PS at 37℃ in a 5% CO2 atmosphere. Recombinant human TRAIL (rhTRAIL) was a gift from T. H. Kim (Department of Biochemistry and Molecular Biology, Chosun University, Korea [14]). MDL-12330A, NKY80 and NB001 were purchased from Sigma (MO) and caspase inhibitors were purchased from Calbiochem (CA).
EZ-cytox viability assay was performed as followed by manufacturer's protocol. Briefly, cells were plated in the wells of a 96-well plate at a density of 1×104 cells/well, incubated for 24 h, and then treated with drugs for 48 h. The EZ-cytox solution was added to the wells and incubated at 37℃ in a CO2 incubator for the last 2 h of incubation, and the plates were read using an enzyme-linked immunosorbent assay plate reader at 405 nm. The absorbance of the untreated cells was set as 100% and cell survival was expressed as a percentage of this value.
Assessment of apoptotic and non-apoptotic cell was performed using a fluorometric mothed as described previously [15]. Briefly, treated cells were stained with 1 µg/mL Hoechst 33342 and 5 µg/ml PI for 15 min at room temperature in the dark. Both floating and attached cells were collected and centrifuged. The pooled cell pellets were washed with cold phosphate-buffered saline (PBS), fixed in 3.7% formaldehyde on ice, washed and resuspended with PBS, and then a fraction of the suspension was centrifuged in a cytospinner (Thermo Fisher Scientific, MA). Slides were prepared, air dried, mounted in the anti-fade solution, and observed under a fluorescence microscope (DM5000, Leica, Germany) at respective excitation/emission wavelengths of 340/425 nm (Hoechst 33342) and 580/630 nm (PI). Morphological assessments of apoptotic and non-apoptotic death were performed: intact blue nuclei, condensed/fragmented blue nuclei, condensed/fragmented pink nuclei and intact or crushed pink nuclei were considered viable, early apoptotic, late apoptotic and non-apoptotic dead cells, respectively. A total of 500 cells distributed across random microscope viewing fields were counted and the number of apoptotic or non-apoptotic cells was expressed as a percentage of the total number of cells scored.
Equal amounts of protein extracts were electrophoretically separated using 10~12% SDS-PAGE and transferred to a nitrocellulose membrane using a standard technique. Antibodies were used to probe for DR4, DR5 (ProSci, CA), c-EBP beta, p65 (Santa Cruz Biotechnology, CA), and CHOP (Cell Signaling Technology, MA). Anti-α-tubulin (BioGenex, CA) was used as a loading control. Signals were acquired using an Image Station 4000MM image analyzer (Kodak, NY).
For the RNAi experiment, siRNA of DR4, 5'-CUGGAAAGUUCAUCUACUU (dtdt)-3' (sense) and 5'-AAGUAGAUGAACUUUCCAG (dtdt)-3' (anti-sense), DR5, 5'-CAGACUUGGUGCCCUUUG (dtdt)-3' (sense) and 5'-UCAAAGGGCACCAAGUCUG (dtdt)-3' (anti-sense), 5'-control siRNA, 5'-CCUACGCCACCAAUUUCGU (dtdt)-3' (sense) and 5'-ACGAAAUUGGUGGCGUAGG (dtdt)-3' (anti-sense) were purchased from Bioneer (Daejeon, Korea). Cells were individually transfected with siRNA oligonucleotides using an Amaxa Transfection System™ (Basel, Switzerland) and grown for 24 h prior to the drug treatment.
Clonogenic activity was measured according to established procedures with some modifications [16]. For the clonogenic assay, 2.5×105 cells/35 mm dishes were pre-incubated with MDL-12330A or vehicle for 18 h followed by incubation with rhTRAIL for another 6 h. Then, cells were trypsinized, counted, re-plated (2,000 cells/60-mm dish), and maintained at 37℃/5% CO2 for 14 days in a humidified atmosphere. The grown cells were fixed, stained with 0.5% crystal violet, and the images were captured by digital camera.
All numerical data are presented as the mean±SE. All data represent the results of at least three independent experiments. Student's t-test was used to evaluate the differences in means between control and treatment group, and one-way ANOVA was applied to analyze differences caused by gene silencing or combined treatment. Significant difference was assumed at p<0.05.
During our investigation of AC activity in tumor cells, we observed that the AC inhibitor MDL-12330A strongly decreased cell viability in various gastrointestinal cancer cell lines including SNU601, SNU638, and AGS cells, as detected by the EZ-cytox viability assay. Although the IC50 for inhibition of AC is 250 µM, decrease of cell viability was obvious even at low concentrations (10~20 µM). In contrast, cell viability of noncancer cell lines IEC-6 and IEC-18 from gut epithelial cell or BEAS-2B from lung epithelial cell were much less affected by MDL-12330A at the same concentration ranges (Fig. 1A).
In order to follow this further, we examined whether the reduction in cell viability included cell death triggered by MDL-12330A. The occurrence of apoptotic and non-apoptotic cell death was assessed after incubation with 10, 20, or 30 µM MDL-12330A for 24 h using the Hoechst33342/PI double-staining method. Apoptotic and non-apoptotic cell death were evaluated by measuring the percentage of blue condensed or cleaved nuclei using Hoechst33342 staining (apoptotic death) and PI-penetrated red cells without apoptotic features such as nuclear cleavage or condensation (non-apoptotic death). As shown in Fig. 1C, MDL-12330A induced both apoptotic and non-apoptotic cell death in SNU601, SNU638, and AGS gastric cancer cell lines. The total number of dead cells linearly correlated with the dose of MDL-12330A. However, apoptotic cell death was the major form of death at concentrations of MDL-12330A up to 20 µM; at concentrations exceeding 20 µM the number of PI-included cells with abnormal shapes increased, indicating an increase in the proportion of cells undergoing non-apoptotic cell death. In this study, we elected to focus on the MDL-12330A-mediated apoptotic mechanism triggered in response to low doses of MDL-12330A.
The apoptotic process is controlled by a cascade of caspases, which is the central enzymatic route for the apoptotic response. Therefore, to understand the signaling pathways involved in MDL-12330A-induced apoptosis, we examined the effect of various caspase inhibitors on apoptotic body formation. The pan-caspase inhibitor z-VAD-fmk almost completely prevented MDL-12330A-induced apoptosis. The caspase-8 inhibitor z-IETD-fmk and the caspase-3 inhibitor z-DEVD-fmk also significantly inhibited apoptosis. In addition, the suppressing effect of the caspase-4 inhibitor z-LEVD-fmk, although weaker than z-IETD-fmk or z-DEVD-fmk, was still significant. In contrast, the caspase-9 inhibitor z-LEHD-fmk had little effect on apoptotic body formation (Fig. 3). These results indicate that both the extrinsic apoptotic pathway and endoplasmic reticulum (ER)-mediated signaling play important roles in MDL-12330A-triggered apoptosis.
Since the extrinsic apoptotic pathway seems to play an important role in MDL-12330A-induced apoptosis, we then explored whether membrane death receptors are involved in the apoptotic mechanism. When we measured the levels of mRNAs encoding the apoptosis-inducing death receptors, DR4 and DR5, in SNU601 cells, a concentration-dependent increase in the mRNA expression of DR5, but not DR4, was observed. In agreement with this result, the protein level of DR5 was also strongly induced in all three GC cell lines upon exposure to MDL-12330A. In order to confirm the role of DR5 in MDL-12330A-induced apoptotic cell death, we knocked down the expression of DR5 using a small interference RNA specifically targeting DR5 and examined the ability of MDL-12330A to induce apoptosis. Upon exposure of SNU601 cells to 20 µM MDL-12330A, the apoptotic rate was approximately 27%; silencing of DR5 partially inhibited apoptosis, whereas, as expected, DR4 silencing had no effect on apoptosis. It should be noted that DR5 silencing only partially prevented apoptosis induced by 20 µM MDL-12330A (about 34.4%), whereas at a lower concentration of MDL-12330A (10 µM) the effect on apoptosis was more apparent (about 78.1%), although the apoptotic rate induced by 10 µM MDL-12330A was lower than that induced by 20 µM MDL-12330A. These results suggest that at low concentrations of MDL-12330A apoptosis is primarily mediated via a DR5-mediated pathway whereas at higher concentrations of MDL-12330A additional DR5-independent apoptotic pathways also operate.
To search for the factors responsible for mediating MDL-12330A-induced DR5 expression, we selected several candidate proteins based on published data and evaluated their potential role in MDL-12330A-induced DR5 expression using RNA interference. Previously published reports have shown that several transcription factors regulate the expression of DR5 including nuclear factor-kB, p53, and C/EBP homologous protein (CHOP) [17181920]. In this study, we excluded p53 as a possible regulator of DR5 expression since SNU601 and SNU638 cells express mutant p53 proteins. As shown in Fig. 4A, we observed that silencing of CHOP suppressed the MDL-12330A-induced DR5 induction in SNU601 and SNU638 cells. Furthermore, MDL-12330A increased CHOP expression level in both of these cells (Fig. 4B). Therefore, MDL-12330A appears to increase DR5 expression through a CHOP-activated pathway. Since the activation of CHOP is suggested to be regulated by ER stress, we then examined whether MDL-12330A induces ER stress by detecting ER stress markers such as glucose regulate protein (GRP) 78/BiP and PERK. As detected in Fig. 4C, MDL-12330A induced BiP and p-PERK levels in SNU601 and SNU638 cells, indicating that MDL-12330A can activate ER stress response.
Since MDL-12330A is developed to be an AC inhibitor, we explored whether other AC inhibitors can induce similar effects on GC cells. However, cell viability and DR5 expression were not affected in the presence of AC inhibitors NB001 or NKY80 in SNU601 and SNU638 cells (Fig. 5). Furthermore, NB001 or NKY80 did not induce BiP expression in these cells (Fig. 5B). Thus, these results suggest that the anticancer effects and ER stress response induced by MDL-12330A may not result from inhibition of AC activity.
As shown in Figs. 3B and C, a low concentration of MDL-12330A (10 µM) was able to elevate DR5 expression and furthermore there was a specific DR5-mediated effect on apoptosis. These results imply that low doses of MDL-12330A could be used to potentially sensitize cells to TRAIL-induced apoptosis. To evaluate whether a low dose of MDL-12330A could enhance TRAIL-induced apoptosis, we pretreated cells with 5 µM or 10 µM MDL-12330A for 24 h and then treated them with 5 and 10 ng/mL of recombinant human TRAIL (rhTRAIL) for 6 h. Treatment with rhTRAIL alone triggered only a low level of apoptosis, but combination with MDL-12330A significantly increased rhTRAIL-mediated apoptosis (Fig. 6A). Importantly, the enhancement of rhTRAIL-induced apoptosis by MDL-12330A was observed in all three GC cell lines we tested. The combination effect was further confirmed using a clonogenic assay, which measures the proliferative capacity of a single cell to produce a large colony. Combination with a low dose of MDL-12330A further reduced the clonogenicity of rhTRAIL-exposed GC cells (Fig. 6B). Taken together, these results suggest that a low dose of MDL-12330A can act as a potent TRAIL sensitizer.
MDL-12330A is an AC inhibitor widely used to establish the biological roles of AC. In this study, however, we have demonstrated a very different activity of this compound. MDL-12330A reduced cell viability and induced cell death by both apoptotic and non-apoptotic death in GC cells. In addition, MDL-12330A increased DR5 expression, which appeared to play an important role in low-dose MDL-12330A-induced apoptosis in GC cells. Importantly, it appeared that these anticancer effects did not result from inhibition of AC activity since other adenylate cyclase inhibitors such as NB001 and NKY80, had no effect on GC cell viability or DR5 expression.
In the present study, we set out to explore the mechanisms involved in the MDL-12330A-mediated antitumor effect. In particular, we focused on the apoptotic death induced by a low concentration of MDL-12330A because a low dose of the drug is likely to be more beneficial in reducing adverse side-effects, and dose limitation in chemotherapy is always a critical issue in clinical applications. In our studies of the mechanism by which MDL-12330A induces apoptosis, we found that MDL-12330A significantly increased DR5 expression in GC cells, and that low concentration MDL-12330A (10 µM)-induced apoptosis appeared to be principally mediated by the DR5. However, at a higher concentration (20 µM) of MDL-12330A, other pathways beyond up-regulation of DR5 expression appeared to be involved in the apoptotic mechanism, since DR5 silencing had only a limited effect on MDL-12330A-induced apoptosis.
DR5 is an essential death receptor mediating TRAIL-induced apoptosis. TRAIL is currently being evaluated as an anti-tumor agent in clinical studies [2122]. In addition, TRAIL is considered to be safe as a therapeutic agent since it showed less side-effects than other members of the TNF super-family like FasL/Fas and TNFα/TNFR1, which show severe side-effects, such as lethal septic shock-like responses and oncogenic NF-kB activation. Pre-clinical investigations have also shown that administration of TRAIL reduced the progression of tumor xenografts without apparent systemic toxicity [23].
However, tumor cell resistance to TRAIL through a variety of mechanisms has emerged as an obstacle to its use as a cancer therapy. Therefore, a great deal of effort has been put into developing strategies to overcome TRAIL resistance. To reverse TRAIL resistance, various possibilities exist, such as upregulation of death receptors, overexpression of pro-apoptotic proteins, downregulation of anti-apoptotic proteins, and prevention of expression of key survival factors. Among these approaches, the combination of TRAIL or TRAIL receptor agonists with TRAIL-sensitizers has been suggested as an efficient way to restore TRAIL sensitivity [2425]. In fact, the effects of various synthetic drugs or compounds derived from natural products have been extensively studied and shown to promote TRAIL-mediated apoptosis in in vitro systems. In the present study, we took notice of the ability of MDL-12330A to induce DR5 expression, and evaluated whether this effect could sensitize TRAIL activity. As expected, the combination of rhTRAIL with low-dose MDL-12330A markedly enhanced rhTRAIL-induced apoptosis in GC cells. Furthermore, the combined treatment significantly reduced the clonogenic activity of these cells, implying that MDL-12330A is a promising candidate to be a novel TRAIL-sensitizer for the treatment of GC.
In an attempt to understand the mechanism involved in DR5 induction, we found that CHOP, a transcription factor in the C/EBP family, plays an essential role in MDL-12330A-mediated DR5 expression. To date, numerous stimuli have been reported to induce CHOP binding to the DR5 promoter thereby elevating DR5 expression [2627]. Activation of CHOP has been shown to be linked to endoplasmic reticulum (ER)-mediated stress signaling. Indeed, MDL-12330A induced expression or activation of ER stress marker, GRP78 and PERK, indicating that MDL-12330A triggers ER stress response. The ER is a major homeostatic organelle that fulfills critical cellular functions, including protein folding, lipid biosynthesis, calcium regulation and redox homeostasis [28]. In the lumen of the ER, proper protein folding is supported by chaperones and folding enzymes. Under physiological conditions, ER misfolded protein load and folding capacity of ER is equilibrated. However, perturbation of ER homeostasis due to accumulation of misfolded proteins, alterations in the calcium homeostasis or loss of the redox balance of the ER lead to a characteristic stress response known as the unfolded protein response [29]. Although, the mechanism underlying MDL-12330A-induced ER stress response should be further clarified, recent data that MDL-12330A blocks voltage-dependent K+ channels in pancreatic beta cells [12] suggest that MDL-12330A may influence ER function through alteration of intracellular ion homeostasis. Indeed, a number of K+ channels and exchangers in the ER membrane have recognized as an essential contributor to ER luminal homeostasis by regulating the ion currents and potential across intracellular membrane, electroneutrality and organelle volume [3031]. In accordance with this, we observed that MDL-12330A-induced apoptosis was reduced by inhibition of caspase-4, which can be activated through the ER stress response. Therefore, the ER stress response appears to be at least partially involved in MDL-12330A-mediated DR5 upregulation and apoptosis. These results agree with previous studies suggesting that ER stress-mediated DR5 induction plays a critical role in the sensitization of TRAIL-resistant cells [273233].
Taken together, these results suggest that MDL-12330A could be used as a potential sensitizer to TRAIL in GC treatment, although further clinical studies are required.
Figures and Tables
Fig. 1
MDL-12330A induces an antitumor effect in human GC cells.
(A, B) Cells were treated with the indicated concentrations of MDL-12330A (MDL) for 24 h and subjected to the EZ-cytox assay for measurement of cell viability (A), or stained with Hoechst 33342/PI to assess the number of cells undergoing apoptotic or non-apoptotic cell death (B) as described in the Methods section. *p<0.05, **p<0.01 vs. control.

Fig. 2
Apoptosis triggered by the combined action of MDL-12330A and rhTRAIL involves activation of caspase-3, caspase-8, and caspase-4, but not caspase-9.
SNU601 and AGS cells were treated with 20 µM MDL-12330A in the absence or presence of z-DEVD, z-IETD, z-LEHD, z-LEVD, or z-VAD for 24 h. The treated cells were stained with Hoechst33342, and apoptotic body was counted under a fluorescence microscope. The number of apoptotic cells was expressed as a percentage of the total number of cells counted. *p<0.05 vs. control; #p<0.05 vs. MDL-12330A (MDL) treated cells.

Fig. 3
DR5 plays an important role in MDL-12330A-mediated apoptosis.
(A) SNU601 cells were exposed to 20 µM MDL-12330A, and the mRNA level of DR5 was determined using real-time PCR. (B) SNU601, SNU638, and AGS cells were treated with various concentrations of MDL-12330A for 24 h, and then analyzed by immunoblotting with antibodies against DR5, DR4, or α-tubulin. (C) SNU601 cells transfected with scrambled small interfering RNA (CTL RNAi), DR5 RNAi or DR4 RNAi were treated with 20 µM MDL-12330A for 24 h (left graph), or 10 µM MDL-12330A for 24 h (right graph). Treated cells were then stained using Hoechst 33342, and apoptotic body was counted under a fluorescence microscope. The number of apoptotic cells was expressed as a percentage of the total number of cells counted. *p<0.05 vs. control; #p<0.05 vs. MDL-12330A treated and CTL RNAi cells.
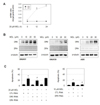
Fig. 4
MDL-12330A-mediated DR5 expression is regulated by CHOP.
(A) SNU601 or SNU638 cells were transfected with a scrambled small interfering RNA (CTL RNAi), p65 RNAi, CHOP RNAi, and C-EBPβ RNAi, and then treated with 20 µM MDL-12330A (MDL) for 24 h. Cell lysates were prepared and analyzed by immunoblotting to assess DR5 expression. Silencing effect of each siRNA was confirmed by immunoblotting in vehicle treated control samples. (B, C) SNU601 or SNU638 cells were exposed to the indicated concentrations of MDL-12330A for 16 h and cell lysates were analyzed by immunoblotting with an antibody to CHOP (B), and to BiP and p-PERK (C). Alpha-tubulin was used as a loading control.

Fig. 5
Antitumor effect of MDL-12330A is not linked to inhibition of adenylate cyclase activity.
(A) SNU601 and SNU638 cells were exposed to other type of adenylate cyclase inhibitors NKY80 and NB001 at indicated concentrations for 24 h, and cell viability was assessed by the EZ-cytox assay. (B) SNU601 and SNU638 cells were incubated with NKY80 or NB001 at 0, 10, 30 and 50 µM for 24 h, and cell lysates were prepared and analyzed by immunoblotting with an antibody to DR5 and BiP.

Fig. 6
MDL-12330A enhances rhTRAIL-induced apoptosis and clonogenicity.
(A) Cells were pre-treated with 5 or 10 µM MDL-12330A for 24 h and then further exposed to rhTRAIL for 6 h. Apoptosis was detected by staining cells with Hoechst 33342 and assessing the ratio of apoptotic nuclei to normal nuclei under a fluorescence microscope. (B) Cells were incubated in the absence or presence of 5 µM MDL-12330A for 24 h and then exposed to 0, 5, or 10 ng/ml rhTRAIL for the last 2 h. Following this, 2×103 cells were re-plated on 60mm dishes. Colonies were stained with crystal violet and counted at 2 weeks post-incubation. #p<0.05 vs. rhTRAIL treated alone.
ACKNOWLEDGEMENTS
We thank Prof. Tae-Hyoung Kim for the kind gift of rh TRAIL, and Ms. Jeong-Eun Choi and Ms. Yoo-Ri Choi for their excellent technical assistance.
Notes
References
1. Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999; 83:18–29.
2. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001; 51:15–36.
3. Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000; 26:243–255.
4. Gura T. How TRAIL kills cancer cells, but not normal cells. Science. 1997; 277:768.
5. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998; 17:3261–3270.
6. Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002; 2:420–430.
7. Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia. 2001; 3:535–546.
8. Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011; 286:5546–5557.
9. Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008; 27:2055–2063.
10. Szliszka E, Krol W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur J Cancer Prev. 2011; 20:63–69.
11. Seifert R, Lushington GH, Mou TC, Gille A, Sprang SR. Inhibitors of membranous adenylyl cyclases. Trends Pharmacol Sci. 2012; 33:64–78.
12. Li X, Guo Q, Gao J, Yang J, Zhang W, Liang Y, Wu D, Liu Y, Weng J, Li Q, Zhang Y. The adenylyl cyclase inhibitor MDL-12,330A potentiates insulin secretion via blockade of voltage-dependent K(+) channels in pancreatic beta cells. PLoS One. 2013; 8:e77934.
13. Gadea A, López E, López-Colomé AM. The adenylate cyclase inhibitor MDL-12330A has a non-specific effect on glycine transport in Müller cells from the retina. Brain Res. 1999; 838:200–204.
14. Shin JN, Park SY, Cha JH, Park JY, Lee BR, Jung SA, Lee ST, Yun CW, Seol DW, Kim TH. Generation of a novel proform of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein that can be reactivated by matrix metalloproteinases. Exp Cell Res. 2006; 312:3892–3898.
15. Kim CH, Han SI, Lee SY, Youk HS, Moon JY, Duong HQ, Park MJ, Joo YM, Park HG, Kim YJ, Yoo MA, Lim SC, Kang HS. Protein kinase C-ERK1/2 signal pathway switches glucose depletion-induced necrosis to apoptosis by regulating superoxide dismutases and suppressing reactive oxygen species production in A549 lung cancer cells. J Cell Physiol. 2007; 211:371–385.
16. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006; 1:2315–2319.
17. Kong F, You H, Zhao J, Liu W, Hu L, Luo W, Hu W, Tang R, Zheng K. The enhanced expression of death receptor 5 (DR5) mediated by HBV X protein through NF-kappaB pathway is associated with cell apoptosis induced by (TNF-α related apoptosis inducing ligand) TRAIL in hepatoma cells. Virol J. 2015; 12:192.
18. Wu GS, Burns TF, McDonald ER 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, el-Deiry WS. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997; 17:141–143.
19. Pennati M, Sbarra S, De Cesare M, Lopergolo A, Locatelli SL, Campi E, Daidone MG, Carlo-Stella C, Gianni AM, Zaffaroni N. YM155 sensitizes triple-negative breast cancer to membrane-bound TRAIL through p38 MAPK- and CHOP-mediated DR5 upregulation. Int J Cancer. 2015; 136:299–309.
20. Lim JH, Park JW, Choi KS, Park YB, Kwon TK. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis. 2009; 30:729–736.
21. Mérino D, Lalaoui N, Morizot A, Solary E, Micheau O. TRAIL in cancer therapy: present and future challenges. Expert Opin Ther Targets. 2007; 11:1299–1314.
22. Fulda S. Tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL). Adv Exp Med Biol. 2014; 818:167–180.
23. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999; 104:155–162.
24. Zhou Y, Tian L, Long L, Quan M, Liu F, Cao J. Casticin potentiates TRAIL-induced apoptosis of gastric cancer cells through endoplasmic reticulum stress. PLoS One. 2013; 8:e58855.
25. Kim S, Lee TJ, Leem J, Choi KS, Park JW, Kwon TK. Sanguinarine-induced apoptosis: generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J Cell Biochem. 2008; 104:895–907.
26. Yoshida T, Shiraishi T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Proteasome inhibitor MG132 induces death receptor 5 through CCAAT/enhancer-binding protein homologous protein. Cancer Res. 2005; 65:5662–5667.
27. Shiraishi T, Yoshida T, Nakata S, Horinaka M, Wakada M, Mizutani Y, Miki T, Sakai T. Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells. Cancer Res. 2005; 65:6364–6370.
28. English AR, Zurek N, Voeltz GK. Peripheral ER structure and function. Curr Opin Cell Biol. 2009; 21:596–602.
29. Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012; 151:217–219.
30. Szewczyk A. The intracellular potassium and chloride channels: properties, pharmacology and function (review). Mol Membr Biol. 1998; 15:49–58.
31. Kuum M, Veksler V, Kaasik A. Potassium fluxes across the endoplasmic reticulum and their role in endoplasmic reticulum calcium homeostasis. Cell Calcium. 2015; 58:79–85.
32. Liu H, Jiang CC, Lavis CJ, Croft A, Dong L, Tseng HY, Yang F, Tay KH, Hersey P, Zhang XD. 2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human melanoma cells through XBP-1-mediated up-regulation of TRAIL-R2. Mol Cancer. 2009; 8:122.
33. Moon DO, Park SY, Choi YH, Ahn JS, Kim GY. Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: involvement of ROS-dependent ER-stress. Biochem Pharmacol. 2011; 82:1641–1650.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download