Abstract
Activation of protein kinase C (PKC) is closely linked with endothelial dysfunction. However, the effect of PKCβII on endothelial dysfunction has not been characterized in cultured endothelial cells. Here, using adenoviral PKCβII gene transfer and pharmacological inhibitors, the role of PKCβII on endothelial dysfucntion was investigated in cultured endothelial cells. Phorbol 12-myristate 13-acetate (PMA) increased reactive oxygen species (ROS), p66shc phosphorylation, intracellular adhesion molecule-1, and monocyte adhesion, which were inhibited by PKCβi (10 nM), a selective inhibitor of PKCβII. PMA increased the phosphorylation of CREB and manganese superoxide dismutase (MnSOD), which were also inhibited by PKCβi. Gene silencing of CREB inhibited PMA-induced MnSOD expression, suggesting that CREB plays a key role in MnSOD expression. Gene silencing of PKCβII inhibited PMA-induced mitochondrial ROS, MnSOD, and ICAM-1 expression. In contrast, overexpression of PKCβII using adenoviral PKCβII increased mitochondrial ROS, MnSOD, ICAM-1, and p66shc phosphorylation in cultured endothelial cells. Finally, PKCβII-induced ICAM-1 expression was inhibited by Mito-TEMPO, a mitochondrial ROS scavenger, suggesting the involvement of mitochondrial ROS in PKC-induced vascular inflammation. Taken together, the results suggest that PKCβII plays an important role in PMA-induced endothelial dysfunction, and that the inhibition of PKCβII-dependent p66shc signaling acts as a therapeutic target for vascular inflammatory diseases.
Cardiovascular risk factors cause the mitochondrial dysfunction that precedes cellular dysfunction [123]. Increased mitochondrial reactive oxygen species (ROS) production is associated with an increase in the expression of adhesion molecules, an early state of vascular disease [456]. Endothelial activation is a pro-inflammatory state of the endothelial cells lining the lumen of blood vessels and is characterized by the elevated expression of cell-surface adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) [7]. This activation is a crucial event in the pathological process of vascular inflammation, the primary cause of cardiovascular diseases such as atherosclerosis [8]. Especially, endothelial mitochondria are critical targets of oxidative stress and play a crucial role in mediating cellular responses [910].
Activation of protein kinase C (PKC) is associated with vascular endothelial dysfunction. Phorbol 12-myristate 13-acetate (PMA), a potent activator of protein kinase C (PKC), disrupts the mitochondrial membrane potential and increases the generation of mitochondrial ROS, leading to mitochondrial dysfunction [11]. Vascular disorders are associated with the activation of PKC signaling by compounds such as PMA, which results in the dysfunction of the endothelial cell barrier [12] and attenuates acetylcholine-induced vasodilation [13]. The p66shc adaptor protein controls oxidtive stress, including the generation of mitochondiral ROS. Under conditions of oxidative stress, p66shc specifically phosphorylated at serine 36 residue which is regulated by PKCβ [1415], leading to mitochondrial ROS production [15].
Mitochondrial superoxide scavenging protein such as MnSOD has a protective function against mitochondrial oxidative stress and its expression is controlled with several transcriptional factors. cAMP-responsive element (CRE)-binding protein (CREB) is nuclear transcriptional factor, CRE is present in the promoter site of MnSOD [16] and PKC-mediated gene expression is dependent on CREB phosphorylation of serine residue at 133 necessary for the transcriptional activation [17].
In endothelial cells, activation of PKCβ inhibits endothelial nitric oxide synthase regulation by insulin, which causes endothelial dysfunction [18]. Among PKC isoenzymes, PKCβII is involved in the pathogenesis in cardiovascular disorder [19]. Previous reports have shown that oxidized low density lipoproteins induced PKCβII membrane translocation and phosphorylation whereas no change in translocation was observed for other PKC isoforms including PKCβI [2021]. However, the biological role of PKCβII in endothelial dysfunction has not been extensively studied. Here, using adenoviral PKCβII gene transfer and pharmacological inhibitors, the role of PKCβII on the endothelial dysfucntion in cultured endothelial cells was evaluated.
PMA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Anilino-monoindolylmaleimide (3-(1-(3-imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, a PKCβ-specific inhibitor (PKCβi), was purchased from Calbiochem (La Jolla, CA, USA). Triphenylphosphonium chloride (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) (Mito-TEMPO), a mitochondrial-specific antioxidant, was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Specific antibodies against ICAM-1, GAPDH, PKCβII (Santa Cruz Biotechnology, CA), phospho-ser36-p66SHC (Calbiochem, CA), SHC (BD Biosciences, NJ), β-actin, Flag (Sigma, MO), phospho-ser133-CREB, CREB (Cell Signaling, MA), and MnSOD (Enzo Life Sciences, NY) were used in this study.
An adenovirus encoding full-length human PKCβII (AdPKCβII) was generated by homologous recombination, as described previously [21]. Human embryonic kidney 293A cells were cultured until an 80% cytopathic effect was observed, and then harvested to prepare the stock recombination adenovirus. The adenovirus was propagated in 293A cells and subsequently purified using the CsCl2 gradient technique, as described previously [22]. To overexpress PKCβII in endothelial cells, the cells were infected with a multiplicity of infection (MOI) of 100 for 24 h. Adβgal was used as an adenoviral control.
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (Cambrex Bio Science, USA), and were cultured and maintained in endothelial growth medium. Cells were used between passages 3 and 6. Small interfering RNA (siRNA) oligonucleotides against human PKCβII and a control with scrambled siRNA sequences were purchased from Santa Cruz Biotechnology (USA). HUVEC cells were transfected with either 100 nM PKCβII-specific or scrambled siRNA. The siRNAs and Lipofectamine® 2000 (Invitrogen, USA) were diluted separately in OptiMEM medium (Life Technologies, USA) and incubated for 5 min at room temperature. Next, siRNA/Lipofectamine® mixtures were incubated together for 20 min before use in the transfection of the HUVEC cells. Cells were incubated at 37℃ in a 5% CO2 incubator for 48 h for gene knockdown.
To determine protein expression levels, 2×105 cells were harvested using 100 µl lysis buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, phosphatase-inhibitor cocktail, and protease-inhibitor cocktail). Cell homogenates (30 µg) were separated by 10 or 12% SDS-PAGE and electrotransferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% skim milk for 1 h at room temperature, blots were incubated overnight at 4℃ with specific primary antibodies (1:1000 anti-ICAM-1, SHC, p-CREB, CREB, PKCβII, GAPDH, or MnSOD, 1:2000 anti-p-p66SHC, and 1:5000 anti-β-actin or anti-Flag), which were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies. Blots were developed for visualization using an enhanced chemiluminescence detection kit (Pierce, Rockford, IL).
U937 cells were fluorescently labeled with 2', 7'-bis-(2-carboxyethyl)-5-(and-6)-carboxy-fluorescein acetoxymethyl ester (BCECF-AM) for the quantitative adhesion assay. U937 cells were fluorescently labeled by incubating the cells (1×107 cells/ml) with 1 µM BCECF-AM in a Hanks Balanced Salt Solution (HBSS) buffer for 30 min at 37℃ and 5% CO2. Monocyte adhesion was quantified by measuring fluorescence (excitation 485 nm/emission 535 nm).
Mitochondrial superoxide production was assessed using MitoSox red (Molecular Probes), a mitochondrion-specific hydroethidine-derivative fluorescent dye that undergoes oxidation to form the DNA-binding red fluorophore ethidium bromide. After 6 h PMA exposure or 24 h PKCβII overexpression, cells were incubated with 5 µM MitoSox red for 10 min in a CO2 incubator. Fluorescence was measured at 590 nm. The data are presented as fold-changes in the mean intensity of MitoSox fluorescence relative to controls.
Elevated mitochondrial ROS generation is related to an increase of adhesion molecules such as ICAM-1 [5]. We examined the effect of PKCβII inhibition on PMA-induced ICAM-1 expression and monocyte adhesion in cultured endothelial cells. As shown in Fig. 1A, PMA treatments resulted in a marked increase in ICAM-1 expression, it expression was reduced by PKCβII inhibition with PKCβi. PKCβII expression is not changed by the treatment of PMA or PKCβi. As shown in Fig. 1B, pretreatment of HUVECs with PKCβi downregulated monocyte adhesion compared to treatment with PMA alone. The above results suggested that PKCβII is involved in PKC-induced endothelial activation.
PKC activation induces mitochondrial ROS generation, leading to mitochondrial dysfunction in endothelial cells [11]. We investigated the effect of PKCβII inhibition on PMA-induced mitochondrial ROS production in endothelial cells. As shown in Fig. 1C, pretreatment with PKCβi (10 nM), a specific PKCβII inhibitor, prevented PMA-induced mitochondrial ROS production. Phosphorylation of p66shc at serine 36 is known to cause mitochondrial ROS generation [23]. As shown in Fig. 1D, PMA-induced p66shc phosphorylation was decreased by PKCβII inhibition.
The phosphorylation of CREB at serine 133 is known to be associated with MnSOD expression [16], which is considered as the primary defensive enzyme against oxidative stress within mitochondria. To explore whether PKCβII can induce CREB phosphorylation, we examined the effect of PKCβi on PMA-induced CREB phosphorylation. As shown in Fig. 2A, CREB phosphorylation (S133) was induced by PMA within 5 min, and remained for 60 min. Total CREB expression was not affected by PMA in HUVECs. PMA-induced CREB phosphorylation was inhibited by PKCβII inhibition (Fig. 2B). We also determined the effect of PKCβi on MnSOD expression in response to PMA. As shown in Fig. 2C, pretreatment with PKCβi downregulated MnSOD expression compared to treatment with PMA alone, suggesting PKCβII-induced MnSOD induction. Furthermore, we investigated whether PMA-induced MnSOD expression is regulated by gene silencing of CREB. Even gene silencing of CREB did not affect basal MnSOD expression, PMA-induced MnSOD expression is markedly inhibited by gene silencing of CREB (Fig. 2D). Collectively, these results indicated that PKCβII activation caused MnSOD expression via CREB phosphorylation at serine 133 in vascular endothelial cells.
To further examine the role of PKCβII, we investigated whether downregulation of PKCβII affected PMA-induced mitochondrial ROS generation and ICAM-1 expression. PMA-induced mitochondrial ROS generation was compared in cells transfected with PKCβII-specific siRNA or control siRNA. As shown in Fig. 3A, mitochondrial ROS production in response to PMA was decreased in PKCβII-targeting siRNA-transfected cells when compared with those transfected with control siRNA. We also analyzed the expression of MnSOD and ICAM-1 in response to PMA in siPKCβII-transfected cells. PMA-induced the expression of MnSOD and ICAM-1 was decreased in PKCβII-downregulated cells relative to that in cells transfected with control siRNA (Fig. 3B).
Having determined that downregulation of PKCβII inhibited PMA-induced mitochondrial ROS and ICAM-1 levels, we investigated the effect of PKCβII overexpression using adenoviral PKCβII gene transfer in HUVECs. After introduction of AdPKCβII or Adβgal for 24 h, mitochondrial ROS level was analyzed. Interestingly, mitochondrial ROS in adenoviral PKCβII-overexpressing cells was significantly elevated relative to that in Adβgal-infected cells (Fig. 4A). The phosphorylation of p66shc at serine 36 residues was not detected in Adβgal-infected cells, however, its phosphorylation was dramatically elevated in AdPKCβII-infected cells (Fig. 4B). These results suggest that specific PKCβII activation leads to mitochondrial oxidative stress. Next, we investigated whether PKCβII overexpression induced endothelial activation in HUVECs. Endothelial activation was assessed by Western blot for ICAM-1. Interestingly, PKCβII overexpression using AdPKCβII led to an increase in the ICAM-1 level in an MOI-dependent manner (Fig. 4C). To confirm the involvement of mitochondrial ROS in PKCβII-induced endothelial activation, we assessed the effect of Mito-TEMPO, a specific mitochondrial antioxidant, in PKCβII overexpression-induced ICAM-1 expression. As shown in Fig. 4D, pretreatment of HUVECs with 10-500 nM Mito-TEMPO significantly inhibited PKCβII-induced ICAM-1 expression in a dose-dependent manner. Also, PKCβII overexpression significantly up-regulated the level of MnSOD in an MOI-dependent manner (0-100 MOI) (Fig. 4E). These results suggest that PKCβII-induced endothelial activation is regulated by mitochondrial ROS.
Utilizing both inhibitors and siRNA, results demonstrated that PKCβII mediates endothelial dysfunction via mitochondrial ROS. Reciprocally, PKCβII overexpression using adenoviral gene transfer induces endothelial dysfunction in endothelial cells. These results demonstrate that PKCβII activation plays an important role in endothelial dysfunction via mitochondrial ROS production in vascular endothelial cells.
Protein kinase Cβ (PKCβ) is a member of the classical PKC family which requires calcium, phospholipids, and diaglyccerol for its activation. PKCβI and PKCβII isoforms are encoded by the same PKCβ gene, but expressed from alternative splicing of C-terminus exons [2425]. The PKCβ inhibitor, an anilino-monoindolylmaleimide compound, acts as a potent, cell-permeable, reversible, and ATP-competitive inhibitor of PKCβ isozymes (IC50=5 nM and 21 nM for human PKCβII and βI, respectively) [26]. In the present study, we used 10 nM of a PKCβ inhibitor to specifically inhibit PKCβII. This pharmacological approach suggested that PMA-induced endothelial dysfunction and mitochondrial activation is closely related with PKCβII.
Activation of PKCβ is involved in mitochondiral oxidative damage. The p66shc adaptor protein controls oxidtive stress, including the generation of mitochondiral ROS. Under conditions of oxidative stress, p66shc is specifically phosphorylated at its serine 36 residue, a process that is regulated by PKCβ [1415], leading to mitochondrial ROS production [15]. In endothelial cells, the activation of PKCβ inhibits endothelial nitric oxide synthase regulation, which causes endothelial dysfunction [182728]. Angiotensin II elicited significantly increased phosphorylation of p66shc in endothelial cells [29]. Inhibition of p66shc activation suppresses angiotensin II-induced vasoconstriction and blood pressure [30]. Overexpression of apurinic/apyrimidinic endonuclease 1 inhibited PKC-induced p66shc phosphorylation which mediates ROS production in endothelial cells [21], and it also can exacerbate atherosclerosis in the aorta [31].
CREB is transcriptional factor that binds to cAMP response elements. Phosphorylation of CREB at serine 133 evoked cAMP response elements-dependent transcription [32]. However, CREB can be activated by other kinases besides protein kinase A (PKA). Among the kinases, PKC activates CREB phosphorylation. Especially, PKC induced the transcriptional activation of MnSOD mRNA, which is mediated by PKC-dependent CREB [1633]. MnSOD protein expression was elevated in response to a PKC agonist, 12-O-tetradecanoylphorbol-13-acetate or TNF-α, but not to hydrogen peroxide [33]. Our data suggested that PMA-induced MnSOD protein expression was significantly inhibited by gene silencing of CREB, suggesting that it is most likely mediated by CREB activation. PMA-induced CREB activation and MnSOD expression are suppressed by PKCβi, suggesting involvement of PKCβII. Cellular oxidative level is regulated by redox balance of oxidation and anti-oxidation. Mitochondrial p66shc is known to play a prooxidant and proapoptoic role in cells [34]. PKCβII can activate p66shc phosphorylation to trigger intracellular ROS generation as pro-oxidant PKC signaling. Chen et al. (2014) reported that ruboxistaurin, PKCβ2 inhibitor, attenuates the adaptor p66shc-mediated intestinal ischemia reperfusion injury [35]. In addition, PKCβII also can regulate CREB to induce MnSOD expression for minimizing undesirable mitochondrial oxidative stress as compensatory anti-oxidant PKC signaling. Therefore, pro-oxidant PKCβII-dependent p66shc signaling and anti-oxidant PKCβII-dependent CREB signaling is counteracted to maintain the endothelial ROS homeostasis.
Mito-TEMPO is widely used a mitochondrial ROS scavenger in intact cells and scavenging mitochondrial ROS could improve mitochondrial function in conditions such as hypertension [36]. Mitochondrial ROS is involved in regulating endothelial adhesion molecules or pro-inflammatory mediators [37]. Endothelial dysfunction or activation is characterized by elevated cell-surface adhesion molecules, which is a crucial event in vascular inflammation [738]. In the present study, overexpression of PKCβII induced endothelial dysfunction or activation like up-regulated ICAM-1 expression. However, inhibition of mitochondrial ROS by Mito-TEMPO suppressed AdPKCβII-induced endothelial dysfunction, suggesting an important role of mitochondrial ROS in PKCβII signaling. Furthermore, inhibition of PKCβII-dependent signaling could act as a therapeutic target for vascular inflammatory diseases.
Figures and Tables
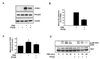 | Fig. 1PKCβII inhibition suppressed PMA-induced mitochondrial ROS generation and endothelial activation in cultured endothelial cells.The effects of PKCβi, a specific PKCβII inhibitor, on PMA-induced (A) ICAM-1 expression, and (B) monocyte adhesion (C), mitochondrial ROS, (D) p66shc phosphorylation are shown. After pretreatment of HUVECs with PKCβi (10 nM), cells were exposed to 250 nM PMA for 6 h (A~C). (A) Following PMA treatment, cell lysates were analyzed by Western blotting for ICAM-1 expression. The β-actin was used as a loading control. (B) Monocyte adhesion was assessed by a monocyte-endothelial cell adhesion assay. Data are expressed as percentage values relative to monocyte adhesion induced by PMA. Each bar represents the mean±S.E.M., n=4. *p<0.05 vs. PMA alone. (C) Relative mitochondrial ROS generation was determined from MitoSOX red fluorescence assays. Fluorescence emission was measured at 590 nm after excitation at 530 nm. Values are expressed as mean fold-change in arbitrary fluorescence values over baseline values. Each bar represents the mean±S.E.M., n=4. #p<0.05 vs. baseline value, *p<0.05 vs. PMA alone. (D) After pretreatment of HUVECs with PKCβi (10 nM), cells were exposed to 250 nM PMA for 5 to 30 min. Phosphorylation of p66shc (S36) and total shc expression were measured by Western blotting.
|
 | Fig. 2PKCβII inhibition suppressed PMA-induced CREB phosphorylation and MnSOD expression in cultured endothelial cells.(A) Induction of CREB phosphorylation by PMA treatment. HUVECs exposed to 250 nM PMA for 5 to 180 min were analyzed by Western blotting for CREB (S133) phosphorylation and total CREB expression. GAPDH was used as a loading control. (B) Effect of PKCβi on PMA-induced phosphorylation of CREB (S133). After pretreatment with PKCβi (10 nM), cells were exposed to 250 nM PMA for 15 min. Phosphorylation of CREB and total CREB expression were measured by Western blotting. (C) Effect of PKCβi on PMA-induced MnSOD expression. After treatment with PKCβi (10 nM), followed by 250 nM PMA for 6 h, MnSOD expression was measured using Western blotting. β-actin was used as a loading control. (D) Effect of down-regulation of CREB on PMA-induced MnSOD expression. After transfection (48 h) with control siRNA or CREB-targeting siRNA (100 nM), cells were exposed to 250 nM PMA for 6 h. Down-regulated CREB using siCREB and PMA-induced MnSOD expression levels were confirmed by Western blotting.
|
 | Fig. 3Down-regulation of PKCβII inhibited PMA-induced mitochondrial ROS generation and ICAM-1 expression.(A) At 48 h post-transfection with control siRNA or PKCβII-targeting siRNA (100 nM), HUVECs were exposed to 250 nM PMA for 6 h. Relative mitochondrial ROS generation was determined from MitoSOX red fluorescence assays. Fluorescence emission was measured at 590 nm after excitation at 530 nm. Values are expressed as the mean fold-change in arbitrary fluorescence values over baseline values. Each bar shows the mean±S.E.M., n=3. #p<0.05 vs. baseline value, *p<0.05 vs. PMA alone. (B) After transfection with control siRNA or PKCβII-targeting siRNA (100 nM), HUVECs were exposed to 250 nM PMA for 3 or 6 h. Down-regulated PKCβII using siPKCβII, and PMA-induced MnSOD and ICAM-1 expressions were analyzed by Western blotting. β-actin was used as a loading control.
|
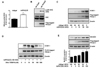 | Fig. 4Overexpression of PKCβII increased mitochondrial ROS generation and ICAM-1 expression.(A) At 24 h post-transduction with Adβgal or AdPKCβII, HUVECs were analyzed for mitochondrial ROS generation using MitoSOX red fluorescence assays. Fluorescence emission was measured at 590 nm with excitation at 530 nm. Values are expressed as the mean fold-change in arbitrary fluorescence values over basal value. Each bar shows the mean±S.E. (n=5). #p<0.05 vs. Adβgal. (B) Effect of PKCβII overexpression on p66shc (S36) phosphorylation. At 24 h post-transduction with Adβgal or AdPKCβII, HUVECs were analyzed for p66shc (S36) phosphorylation and total shc expression using Western blot analysis. (C) ICAM-1 expression in PKCβII-overexpressed HUVECs. Following transduction with Adβgal or AdPKCβII, cells were analyzed using Western blotting for ICAM-1 expression. (D) Effect of Mito-TEMPO, mitochondrial ROS inhibitor, on PKCβII-induced ICAM-1 expression. After treatment with Mito-TEMPO (10~500 nM) in AdPKCβII-overexpressed cells, the change in ICAM-1 level was analyzed by Western blotting. Flag-tagged PKCβII overexpression was confirmed by anti-Flag antibody detection. β-actin was used as a loading control. (E) MnSOD expression in PKCβII-overexpressed HUVECs. Following transduction with Adβgal or AdPKCβII, cells were analyzed using Western blotting for MnSOD expression. Quantitative analysis of MnSOD is shown in bottom of Fig. 4E. The total adenoviral MOI of 100 was balanced in the Adβgal control. Flag-tagged PKCβII overexpression was confirmed by anti-Flag antibody detection. β-actin was used as a loading control. #p<0.05 vs. Adβgal.
|
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A1029617, 2016R1A6A3A11932015). English language usage has been checked by a professional editor who is a native English speaker.
Notes
References
1. Giovannini C, Matarrese P, Scazzocchio B, Sanchez M, Masella R, Malorni W. Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS Lett. 2002; 523:200–206.
2. Munusamy S, MacMillan-Crow LA. Mitochondrial superoxide plays a crucial role in the development of mitochondrial dysfunction during high glucose exposure in rat renal proximal tubular cells. Free Radic Biol Med. 2009; 46:1149–1157.
3. Sommer SP, Sommer S, Sinha B, Wiedemann J, Otto C, Aleksic I, Schimmer C, Leyh RG. Ischemia-reperfusion injury-induced pulmonary mitochondrial damage. J Heart Lung Transplant. 2011; 30:811–818.
4. Chen KH, Reece LM, Leary JF. Mitochondrial glutathione modulates TNF-alpha-induced endothelial cell dysfunction. Free Radic Biol Med. 1999; 27:100–109.
5. Zinovkin RA, Romaschenko VP, Galkin II, Zakharova VV, Pletjushkina OY, Chernyak BV, Popova EN. Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY). 2014; 6:661–674.
6. Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007; 9:301–307.
7. Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013; 123:540–541.
8. Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995; 57:791–804.
9. Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007; 100:1128–1141.
10. Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013; 112:1171–1188.
11. Joo HK, Lee YR, Park MS, Choi S, Park K, Lee SK, Kim CS, Park JB, Jeon BH. Mitochondrial APE1/Ref-1 suppressed protein kinase C-induced mitochondrial dysfunction in mouse endothelial cells. Mitochondrion. 2014; 17:42–49.
12. Patterson CE, Davis HW, Schaphorst KL, Garcia JG. Mechanisms of cholera toxin prevention of thrombin- and PMA-induced endothelial cell barrier dysfunction. Microvasc Res. 1994; 48:212–235.
13. Goel A, Thor D, Anderson L, Rahimian R. Sexual dimorphism in rabbit aortic endothelial function under acute hyperglycemic conditions and gender-specific responses to acute 17beta-estradiol. Am J Physiol Heart Circ Physiol. 2008; 294:H2411–H2420.
14. Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007; 315:659–663.
15. Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005; 122:221–233.
16. Kim HP, Roe JH, Chock PB, Yim MB. Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. J Biol Chem. 1999; 274:37455–37460.
17. Bedogni B, Pani G, Colavitti R, Riccio A, Borrello S, Murphy M, Smith R, Eboli ML, Galeotti T. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003; 278:16510–16519.
18. Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006; 55:691–698.
19. Lei S, Li H, Xu J, Liu Y, Gao X, Wang J, Ng KF, Lau WB, Ma XL, Rodrigues B, Irwin MG, Xia Z. Hyperglycemia-induced protein kinase C β2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes. 2013; 62:2318–2328.
20. Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009; 23:1081–1091.
21. Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, Park JB, Ryoo S, Irani K, Jeon BH. Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 2011; 91:502–509.
22. Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, Berkowitz DE, Irani K. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004; 95:902–910.
23. Sun L, Xiao L, Nie J, Liu FY, Ling GH, Zhu XJ, Tang WB, Chen WC, Xia YC, Zhan M, Ma MM, Peng YM, Liu H, Liu YH, Kanwar YS. p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am J Physiol Renal Physiol. 2010; 299:F1014–F1025.
24. Ono Y, Kurokawa T, Fujii T, Kawahara K, Igarashi K, Kikkawa U, Ogita K, Nishizuka Y. Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett. 1986; 206:347–352.
25. Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008; 88:1341–1378.
26. Tanaka M, Sagawa S, Hoshi J, Shimoma F, Matsuda I, Sakoda K, Sasase T, Shindo M, Inaba T. Synthesis of anilino-monoindolylmaleimides as potent and selective PKCbeta inhibitors. Bioorg Med Chem Lett. 2004; 14:5171–5174.
27. Lee SK, Lee JY, Joo HK, Cho EJ, Kim CS, Lee SD, Park JB, Jeon BH. Tat-mediated p66shc transduction decreased phosphorylation of endothelial nitric oxide synthase in endothelial cells. Korean J Physiol Pharmacol. 2012; 16:199–204.
28. Chiasson VL, Quinn MA, Young KJ, Mitchell BM. Protein kinase CbetaII-mediated phosphorylation of endothelial nitric oxide synthase threonine 495 mediates the endothelial dysfunction induced by FK506 (tacrolimus). J Pharmacol Exp Ther. 2011; 337:718–723.
29. Lee SK, Kim HS, Song YJ, Joo HK, Lee JY, Lee KH, Cho EJ, Cho CH, Park JB, Jeon BH. Alteration of p66shc is associated with endothelial dysfunction in the abdominal aortic coarctation of rats. FEBS Lett. 2008; 582:2561–2566.
30. Kang G, Lee YR, Joo HK, Park MS, Kim CS, Choi S, Jeon BH. Trichostatin a modulates angiotensin II-induced vasoconstriction and blood pressure via inhibition of p66shc activation. Korean J Physiol Pharmacol. 2015; 19:467–472.
31. Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang P, King GL. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-β isoform in the endothelium. Circ Res. 2013; 113:418–427.
32. Kee BL, Arias J, Montminy MR. Adaptor-mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996; 271:2373–2375.
33. Chung YW, Kim HK, Kim IY, Yim MB, Chock PB. Dual function of protein kinase C (PKC) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced manganese superoxide dismutase (MnSOD) expression: activation of CREB and FOXO3a by PKC-alpha phosphorylation and by PKC-mediated inactivation of Akt, respectively. J Biol Chem. 2011; 286:29681–29690.
34. Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002; 21:3872–3878.
35. Chen Z, Wang G, Zhai X, Hu Y, Gao D, Ma L, Yao J, Tian X. Selective inhibition of protein kinase C β2 attenuates the adaptor P66 Shc-mediated intestinal ischemia-reperfusion injury. Cell Death Dis. 2014; 5:e1164.
36. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010; 107:106–116.
37. Yu JH, Kim CS, Yoo DG, Song YJ, Joo HK, Kang G, Jo JY, Park JB, Jeon BH. NADPH oxidase and mitochondrial ROS are involved in the TNF-alpha-induced vascular cell adhesion molecule-1 and monocyte adhesion in cultured endothelial cells. Korean J Physiol Pharmacol. 2006; 10:217–222.
38. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007; 100:460–473.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download