INTRODUCTION
METHODS
Chemicals
Preparation of SC seed oil and methanolic and ethanolic extracts of SC fruits
Chemical isolation
Cell culture
DNA constructs and transient transfection of hTRPV3
Electrophysiology
Intracellular free calcium concentration ([Ca2+]i) measurements
Statistical analysis
RESULTS
Methanolic extract of SC can activate ITRPV3
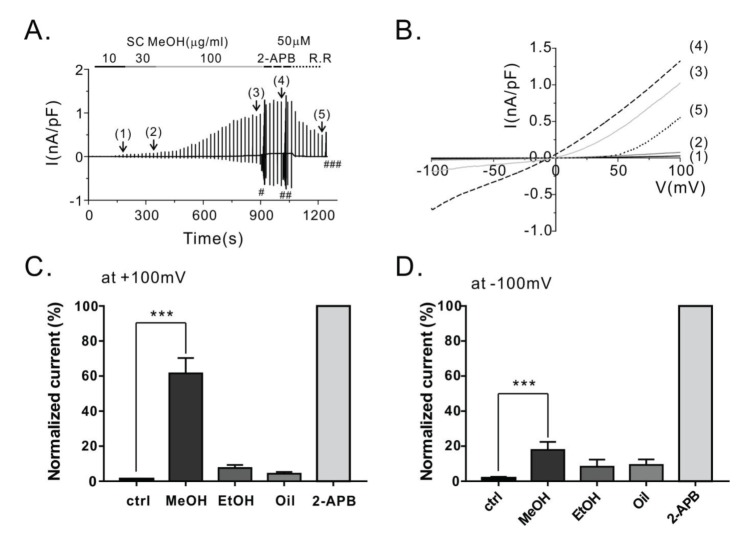 | Fig. 1Effect of methanolic extract of Schisandra chinensis (SCMeOH) on TRPV3 current (ITRPV3) induction in HEK293T cells overexpressing TRPV3 (hTRPV3-HEK293T).(A) Representative patch clamp recordings of ITRPV3 in response to continuous ramp-like voltage pulse in TRPV3-HEK293T cells. In the whole-cell patch clamp recording, ramp-like pulses from −100 to 100 mV (dV/dt=0.04 V/sec) were applied every 20 sec to determine the current-voltage relationship (I~V curves). The numbers in parentheses indicate the steady state ITRPV3 induced by 10 (1), 30 (2), or 100 (3) µg/mL SC fruit extract or 50 µM 2-APB (4), or inhibited by 10 µM ruthenium red (R.R) (5). (B) The corresponding I~V curves at each steady state ITRPV3. (C, D) Summary of normalized inward and outward currents at +100 mV (C) and -100 mV (D) induced by various concentrations of the SC extracts. The current densities were normalized to the maximum ITRPV3 with the 2-APB treatment and used at the end of the experiment. 2-APB, 2-aminoethyl diphenyl borate; SCMeOH, methanolic extract of dried Schisandra chinensis fruits; SCEtOH, ethanolic extract of dried Schisandra chinensis fruits; SCseed oil, Schisandra chinensis seed oil extract. *** indicates p<0.001 vs. the baseline current (control).
|
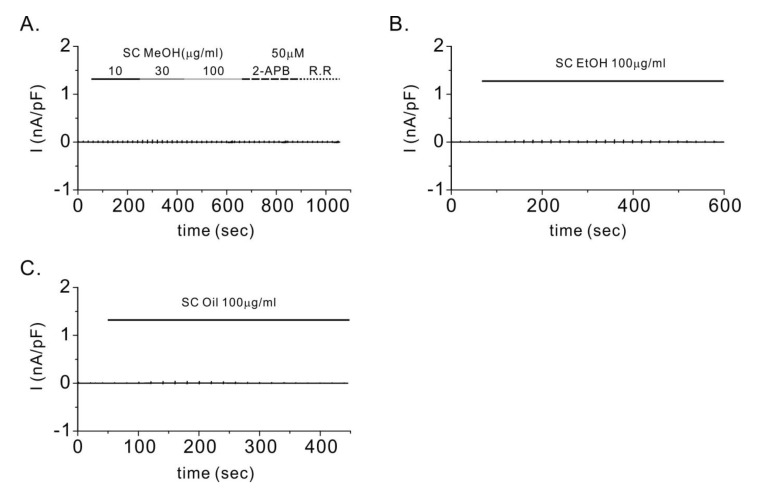 | Fig. 2Control experiments for TRPV3 currents (ITRPV3) in mock-transfected HEK293T cells.To confirm whether Schisandra chinensis (SC) extracts generate non-selective cationic currents, whole-cell patch clamp experiments were done in mock-transfected HEK293T cells (A~C). Representative patch clamp recording of mock-transfected HEK293T cells treated with the methanolic extract of dried Schisandra chinensis fruits (SCMeOH), ethanolic extract of dried SC fruits (SCEtOH), and SC seed oil (SCseed oil). In the case of SCMeOH, we treated the cells with 2-aminoethyl diphenyl borate (2-APB) to activate ITRPV3. We then treated the cells with the TRPV3 blocker ruthenium red (R.R) to inhibit TRPV3. It was observed that no current was generated from the SC extract or 2-APB treatment in the mock-transfected HEK293T cells.
|
Table 1
Effects of Schisandra chinensis (SC) fruit extracts and seed oil on TRPV3 current (ITRPV3)
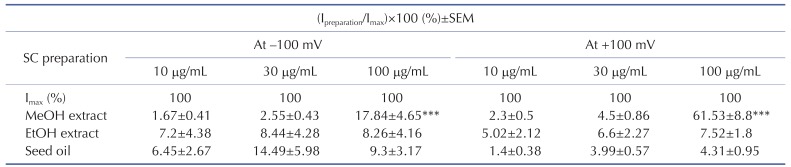
After confirming that there was no basal current, HEK293T cells overexpressing human TRPV3 were treated with the SC extracts and seed oil at concentrations of 10, 30, or 100 µg/mL. The results shown are percentage changes versus the maximum current (Imax), which were obtained by treatment of the cells with 50 µM 2-aminoethyl diphenyl borate, followed by current normalization to 100%. The data are presented as mean±standard error of the mean (SEM) (n=5). ***Indicates p<0.001 vs. the control group. Imax, maximum current; Ipreparation, current induced by SC preparation.
Agonistic effect of γ-schisandrin on ITRPV3
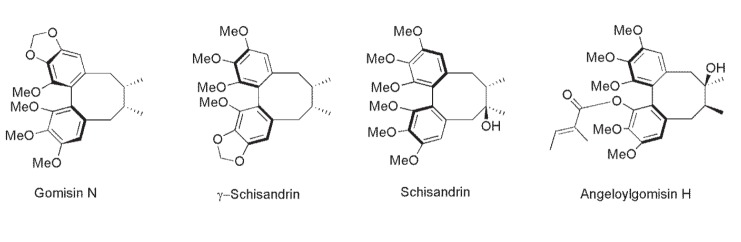 | Fig. 3Structures of the four chemical compounds isolated from the Schisandra chinensis fruit extract. |
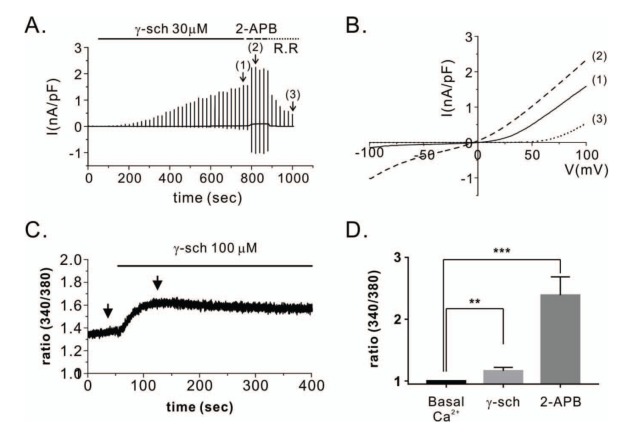 | Fig. 4Effect of γ-schisandrin (γ-Sch) on TRPV3 activation in HEK293T cells overexpressing TRPV3.(A) Representative patch clamp recordings showing TRPV3 current (ITRPV3) activation by 30 µM γ-Sch. At the end of the experiment, 50 µM 2-APB was added to the bath solution in order to determine the maximum current. Following treatment with 10 µM ruthenium red (R.R), a TRPV3 inhibitor, ITRPV3 that was not due to a non-selective cationic current was determined. The numbers in parentheses indicate the corresponding current to voltage relations (I~V curves) depicted in Fig. 1B. (B) I~V relationship curves of steady state ITRPV3 due to 30 µM γ-Sch (1) and 50 µM 2-APB (2) treatments. The 10 µM R.R potently inhibited the inward current of ITRPV3; however, it had relatively no impact on the outward current at the same concentration. (C) Representative trace recordings of intracellular calcium measurements ([Ca2+]i), which were increased by 100 µM γ-Sch in HEK293T cells overexpressing TRPV3 (hTRPV3-HEK293T). (D) Comparison of peaks showing increases in [Ca2+]i induced by 100 µM γ-Sch or 100 µM 2-APB in the hTRPV3-HEK293T cells. The data was normalized by resetting the [Ca2+]i to 1. Data are presented as mean±standard error of the mean (n=8). **indicates p<0.01 and *** indicates p<0.001, each vs. the basal [Ca2+]i. 2-APB, 2-aminoethyl diphenyl borate.
|
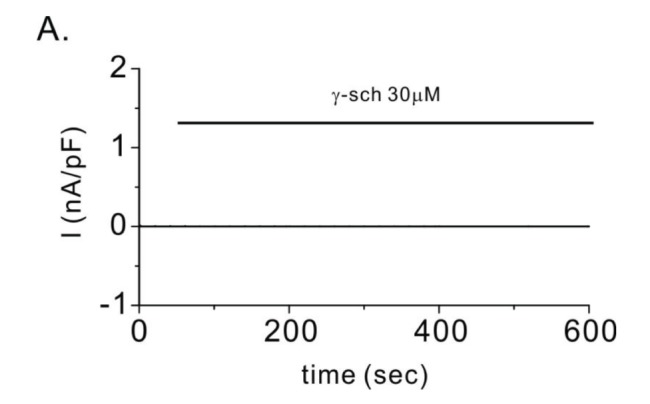 | Fig. 5γ-schisandrin (γ-Sch) generated no additional currents in the mock-transfected HEK293T cells.(A) Representative patch clamp recordings after treatment of the cells with 30 µM γ-Sch.
|
Table 2
TRPV3 currents (ITRPV3) induced by compounds isolated from Schisandra chinensis fruits
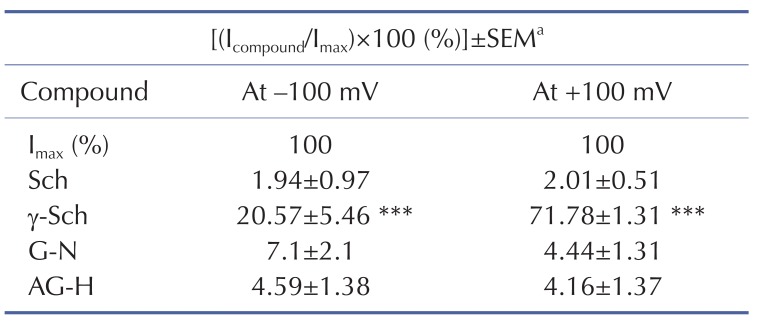
aIndicates the control and is the normalized ITRPV3 after treatment with 50 µM 2-APB at the maximum current (Imax), which was set at 100%, before treatment with the isolated compound (Icompound). The data represent the current relative to Imax and are presented as mean±standard error of the mean (SEM). ***indicates p<0.001 vs. the control group.
Sch, schisandrin; γ-Sch, γ-schisandrin; AG-H, angeloyl gomisin H; G-N, gomisin N; 2-APB, 2-aminoethyl diphenyl borate.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download