Abstract
27-Hydroxycholesterol induces differentiation of monocytic cells into mature dendritic cells, mDCs. In the current study we sought to determine roles of the PI3K and the ERK pathways in the 27OHChol-induced differentiation. Up-regulation of mDC-specific markers like CD80, CD83 and CD88 induced by stimulation with 27OHChol was significantly reduced in the presence of LY294002, an inhibitor of PI3K, and U0126, an inhibitor of ERK. Surface expression of MHC class I and II molecules elevated by 27OHChol was decreased to basal levels in the presence of the inhibitors. Treatment with LY294002 or U0126 resulted in recovery of endocytic activity which was reduced by 27OHChol. CD197 expression and cell adherence enhanced by 27OHChol were attenuated in the presence of the inhibitors. Transcription and surface expression of CD molecules involved in atherosclerosis such as CD105, CD137 and CD166 were also significantly decreased by treatment with LY294002 and U0126. These results mean that the PI3K and the ERK signaling pathways are necessary for differentiation of monocytic cells into mDCs and involved in over-expression of atherosclerosis-associated molecules in response to 27OHChol.
The serum level of 27-hydroxycholesterol (27OHChol), the most abundant oxysterol in atherosclerotic lesions, increases in hypercholesterolemia [12]. 27OHChol initiates or amplifies inflammatory and immunological responses by enhancing recruitment of monocytic cells, migration of CCR5-expressing Th1 lymphocytes, and inducing differentiation of monocytic cells into a mature dendritic cell (mDC) phenotype [345]. 27OHChol up-regulates pattern recognition receptors, like CD14, in monocytic cells [46]. The oxysterol also induces expression of mDC-specific markers like CD80, CD83 and CD88 and atherosclerosis-associated CD antigens including CD105, CD137 and CD166 [5]. Because these responses are important in initiation, progression, and development of atherosclerosis, elucidation of individual signaling pathways in the processes is very helpful to understand pathogenesis of the disease.
Multiple pathways are involved in molecular and cellular effects of 27OHChol. 27OHChol promotes pro-inflammatory processes in an animal model of atherosclerosis and growth of estrogen receptor-positive breast cancer via estrogen receptor (ER)-α [78]. 27OHChol enhances phosphorylation of Akt and ERK of monocytic cells [9]. The PI3K/Akt and ERK pathways are involved in expression of soluble CD14 (sCD14), MMP-9, chemokines, activation of the nuclear factor erythroid 2 p45-related factor 2 (Nrf2), and monocytic cell survival [6910]. These findings indicate that the Akt and ERK are key signaling molecules for 27OHChol-mediated responses of monocytic cells.
In the current study, we investigated involvement of PI3K and ERK in morphological, functional, and molecular changes occurring in DC differentiation in response to 27OHCHol to understand underlying mechanisms leading to increased number of DCs in a milieu rich in cholesterol oxidation products.
THP-1 cells purchased from ATCC were maintained as described [11]. 27OHChol was purchased from Research Plus, Inc. (Bayonne, NJ, USA). Fluorescein isothiocyanate (FITC)-conjugated dextran (40 kDa), LY294002 and U0126 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488-conjugated secondary antibodies for FACS analysis were purchased from Invitrogen (Eugene, OR, USA).
After removal of non-adherent cells by washing with PBS, adherent cells were counted by using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc. Rockville, MD, USA) following manufacturer's instructions [11].
Endocytic activity of cells was measured after uptake of FITC conjugated dextran by using a FACS Canto II (BD Biosciences, San Jose, CA, USA) [11].
Quantitative real-time PCR was performed by using a LightCycler 96 Real-Time PCR System (Roche, Germany), as described [12]. The sequence of CD molecule primers was forward 5′-TGGTGCTGGCTGGTCTTTC and reverse 5′-CTGTGCCACTTCTTTCACTTCC (CD80); forward 5′-TCCTGAGCTGCGCCTACAG and reverse 5′-GCAGGGCAAGTCCACATCTT (CD83); forward 5′-GTGGTCCGGGAGGAGTACTTT and reverse 5′-GCCGTTTGTCGTGGCTGTA (CD88) ; forward 5′-CATCCTTGAAGTCCATGTCCTCTT and reverse 5′-GCCAGGTGCCATTTTGCTT (CD105); forward 5′-TCACTGCCTGGGGGCAGGAT and reverse 5′-GGCGGGGTCACAGAGGATGC (CD137); forward 5′-TCCTGCCGTCTGCTCTTCT and reverse 5′-TTCTGAGGTACGTCAAGTCGG (CD166). Primers for GAPDH were forward 5′-ATGGGGAAGGTGAAGGTCG and reverse 5′-GGGGTCAT TGATGGCAACAATA.
Fluorescence of CD80, CD83, CD88, CD105, CD137, CD166, CD197, and major histocompatibility complex (MHC) class I and II molecules were analyzed by using a FACS Canto II (BD Biosciences, San Jose, CA, USA) (For more information of antibodies, please see the cited reference) [11].
The effects of 27OHChol on phosphorylation Akt and ERK1/2 were determined by ELISA. Phosphorylated forms of Akt and ERK1/2 were elevated after addition of 27OHChol, but the elevation of p-Akt and p-ERK1/2 was blocked in the presence of a PI3K inhibitor, LY294002, and an MEK inhibitor, U0126, which inhibit activation of Akt and ERK1/2, respectively (Supplementary Fig. 1).
We examined whether expression of mDC-specific markers induced by 27OHChol was affected by LY294002 and U0126 (Fig. 1A). Levels of transcripts of CD80, CD83, and CD88 were dramatically increased to 10.9-, 12.5-, and 7.7-fold after stimulation with 27OHChol, respectively, compared with unstimulated control. But, the increases were reduced to basal levels in the presence of the inhibitors.
We examined effects of the inhibitors on surface expression of mDC-specific markers via FACS analysis (Fig. 1B). The percentage of control cells positive for CD80 was 1.2%, and the percentage was increased to 40.2% after stimulation with 27OHChol. But the increase was reduced to 6.3% and 9.7% in the presence of LY294002 and U0126, respectively. The percentage of CD83-positive control cells was increased from 3.6% to 30.8% after 27OHChol stimulation, but the increase was reduced to 4.1% and 10.9% by treatment with LY294002 and U0126, respectively. The percentage of CD88-positive cells was increased from 5.5% to 25.7% after the stimulation, which was reduced to 5.0% and 11.4% in the presence of LY294002 and U0126, respectively. Collectively, these results indicate that 27OHChol necessitated PI3K and ERK for induction of expression of mDCs-specific markers.
We investigated whether the inhibitors influenced expression of MHC class molecules (Fig. 2). The percentage of control cells positive for MHC class I molecule was 6.2% and it increased to 22.0% by stimulation with 27OHChol. Similarly, the percentage of MHC class II-positive control cells was increased from 5.2% to 27.1% by 27OHCHol stimulation. However, the surface levels of both MHC molecules were reduced to those that are comparable to control cells in the presence of LY294200 and U0126. These results indicate that PI3K and ERK were necessary for 27OHChol to induce surface expression of MHC molecules.
We performed endocytic activity tests to determine whether PI3K and ERK are involved in functional alteration of monocytic cells (Fig. 3). The percentage of control cells exhibiting endocytic activity was 17.1%, which was significantly reduced to 3.4% after stimulation with 27OHChol. However, the reduction was recovered to 11.8% and 7.2% in the presence of LY294002 and U0126, respectively. These results mean that PI3K, in comparison with ERK, more affected functional changes of monocytic cells in response to 27OHChol.
CD197 (CCR7) is highly expressed on mDCs stage [13]. We examined effects of the inhibitors on CD197 expression by flow cytometry (Fig. 4A). The percentage of cells positive for CD197 was increased from 5.1% to 13.7% after stimulation with 27OHChol, but the percentage was reduced to 5.7% and 4.3% by treatment with LY294002 and U0126, respectively. We examined whether the inhibitors affected cell adherence (Fig. 4B). The number of adherent cells was increased by 20.4 fold after 27OHChol stimulation, but the increase was reduced by 3.9- and 7.6-fold in the presence of LY294002 and U0126, respectively. These data mean that PI3K and ERK were involved in the morphological changes and over-expression of the molecule involved in migration.
We investigated whether PI3K and ERK participate in expression of CD molecules associated with atherosclerosis. Levels of transcripts of CD105, CD137 and CD166 were assessed by realtime-PCR. Compared with control, transcripts levels of CD105, CD137, and CD166 were elevated by 13.1-, 11.2-, and 14.3-fold by stimulation with 27OHChol, respectively. However, the increases were significantly reduced to basal levels in the presence of LY294002 and U0126 (Fig. 5A).
We also investigated effects of the inhibitors on surface expression of the molecules by flow cytometry (Fig. 5B). The percentage of control cells positive for CD105 was 3.2% and it increased to 16.5% after stimulation with 27OHChol, but the percentage was reduced to 4.4% and 6.1% by treatment with LY294002 and U0126, respectively. The percentage of CD137-positive control cells was increased from 7.6% to 16.4% after 27OHChol stimulation. But the percentage was decreased to 9.5% and 7.8% in the presence of LY294002 and U0126, respectively. The percentage of CD166-positive control cells was increased from 4.7% to 14.7% after the stimulation, which was reduced to 4.0% and 3.2% in the presence of LY294002 and U0126, respectively. These results indicate that PI3K and ERK could mediate expression of CD105, CD137, and CD166 at the transcriptional and protein levels.
Monocytes differentiate into DCs via interleukine-4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent manner [1415], and the differentiation is modulated by multiple signaling pathways [16]. A GM-CSF signal induces activation of PI3K, STAT, MAPK, and NF-κB, which regulate development of DCs. 27OHChol also induces the differentiation of monocytic cells into mDCs [5]. However, it is unknown which signaling pathway is responsible for the differentiation in response to 27OHChol. We presumed that the PI3K/Akt pathway would be involved in 27OHChol-induced differentiation because Akt was activated by 27OHChol [39]. Results of the current study indicate involvement of PI3K in DC differentiation induced by 27OHChol. Taken together, these findings suggest that PI3K/Akt pathway is necessary for differentiation of monocytes into DCs in response to distinct stimuli.
Some surface molecules are highly expressed on stage of mDCs. Levels of CD molecules like CD80, CD83 and CD88, and MHC class I/II molecules are elevated on cell surface during differentiation of immature DCs (imDCs) to mDCs [14]. Expression of the molecules is modulated through multiple signaling pathways, including MAPK, ERK and NF-κB [131415]. We observed reduction of the CD molecules and MHC I/II molecules by treatment with LY294002 and U0126, which means that the PI3K and the ERK pathways affect the 27OHChol-induced differentiation of monocytic cells into mDCs. An increased expression of MHC class molecules and a reduced endocytic activity are major characteristics of mDCs. The mDCs differentiated from monocytes by oxysterols secrete inflammatory cyto-/chemokines like IL-6, IL-8 and TGF-β, which induce recruitments of inflammatory cells [21317]. These results are in line with the previous study that reported regulation of MHC class molecules on cell surface via PI3K/Akt [18].
CD197, a CD molecule highly expressed on mDCs, specifically interacts with CCL19 and CCL21 which are expressed in endothelial cells and in T cell zone of lymphoid organs [192021]. Therefore, CD197 is involved in migration of mDCs into secondary lymph nodes, like spleen. The migrated mDCs stimulate naive T cells in secondary lymph nodes. The results of inhibition of CD197 expression by treatment with LY294002 and U0126 suggest that 27OHChol can affect migration of mDCs and stimulation of T cells via overexpression of CD197 and that the PI3K and the ERK pathways may regulate the migration and T cell activation in the presence of oxysterol molecules.
We also determined expression of CD molecules, such as CD105, CD137 and CD166, involved in progression, acceleration and development of atherosclerosis [1122]. Our results indicate that 27OHChol-induced expression of these molecules is regulated by PI3K and ERK, which agrees with previous study. Lee et al. have reported that CD105 (endoglin) interacts with PI3K subunits and activates PI3K/Akt pathway at angiogenesis [23]. CD137 (4-1BB), part of the tumor necrosis factor receptor superfamily 9 (TNFRSF9), can directly or indirectly promote activity of PI3K and Akt [24]. CD166 (Alcam) is regulated through the PI3K/Akt pathway in liver cancer cells [25]. Taken together, these results suggest possible involvement of the PI3K and the ERK pathways in pathogenesis of atherosclerosis.
The current study sought to investigate and compare involvement of PI3K and ERK in DC differentiation induced by 27OHChol. Results of this study indicate that both the PI3K and the ERK pathways participate in differentiation of monocytic cells into mDCs as well as in over-expression of molecules associated with atherosclerosis in a milieu rich in cholesterol oxidation product.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A4A01010144).
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1C1B1013443).
Notes
References
1. Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999; 142:1–28. PMID: 9920502.

2. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995; 91:2488–2496. PMID: 7729036.
3. Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol Appl Pharmacol. 2014; 274:462–470. PMID: 24370436.

4. Kim SM, Lee SA, Kim BY, Bae SS, Eo SK, Kim K. 27-Hydroxycholesterol induces recruitment of monocytic cells by enhancing CCL2 production. Biochem Biophys Res Commun. 2013; 442:159–164. PMID: 24269812.

5. Son Y, Kim SM, Lee SA, Eo SK, Kim K. Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells. Biochem Biophys Res Commun. 2013; 438:161–168. PMID: 23876312.

6. Kim SM, Kim BY, Eo SK, Kim CD, Kim K. 27-Hydroxycholesterol up-regulates CD14 and predisposes monocytic cells to superproduction of CCL2 in response to lipopolysaccharide. Biochim Biophys Acta. 2015; 1852:442–450. PMID: 25497142.

7. Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor alpha. Cell Metab. 2014; 20:172–182. PMID: 24954418.

8. Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013; 5:637–645. PMID: 24210818.

9. Vurusaner B, Gamba P, Testa G, Gargiulo S, Biasi F, Zerbinati C, Iuliano L, Leonarduzzi G, Basaga H, Poli G. Survival signaling elicited by 27-hydroxycholesterol through the combined modulation of cellular redox state and ERK/Akt phosphorylation. Free Radic Biol Med. 2014; 77:376–385. PMID: 25110320.

10. Vurusaner B, Gamba P, Gargiulo S, Testa G, Staurenghi E, Leonarduzzi G, Poli G, Basaga H. Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic Biol Med. 2016; 91:93–104. PMID: 26689473.

11. Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008; 117:1292–1301. PMID: 18285570.

12. Seo HC, Kim SM, Eo SK, Rhim BY, Kim K. 7α-hydroxycholesterol elicits TLR6-mediated expression of IL-23 in monocytic cells. Biomol Ther (Seoul). 2015; 23:84–89. PMID: 25593648.

13. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18:767–811. PMID: 10837075.

14. Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998; 160:4587–4595. PMID: 9574566.
15. Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997; 27:431–441. PMID: 9045914.
16. van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012; 119:3383–3393. PMID: 22323450.

17. Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab Invest. 2010; 90:970–984. PMID: 20458277.

18. Majmundar AJ, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, Simon MC. O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Biol. 2012; 32:36–49. PMID: 22006022.
19. Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998; 28:2025–2034. PMID: 9645384.

20. Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000; 97:12694–12699. PMID: 11070085.

21. Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008; 29:325–342. PMID: 18799141.

22. Piao M, Tokunaga O. Significant expression of endoglin (CD105), TGFbeta-1 and TGFbeta R-2 in the atherosclerotic aorta: an immunohistological study. J Atheroscler Thromb. 2006; 13:82–89. PMID: 16733295.

23. Lee NY, Golzio C, Gatza CE, Sharma A, Katsanis N, Blobe GC. Endoglin regulates PI3-kinase/Akt trafficking and signaling to alter endothelial capillary stability during angiogenesis. Mol Biol Cell. 2012; 23:2412–2423. PMID: 22593212.

24. So T, Croft M. Regulation of PI-3-kinase and Akt signaling in T lymphocytes and other cells by TNFR family molecules. Front Immunol. 2013; 4:139. PMID: 23760533.

25. Ma L, Wang J, Lin J, Pan Q, Yu Y, Sun F. Cluster of differentiation 166 (CD166) regulated by phosphatidylinositide 3-Kinase (PI3K)/AKT signaling to exert its anti-apoptotic role via yes-associated protein (YAP) in liver cancer. J Biol Chem. 2014; 289:6921–6933. PMID: 24482231.

SUPPLEMENTARY MATERIALS
Supplementary data including three figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp021-03-04-s001.pdf.
Supplementary Fig. 1
Effects of inhibitors of PI3K and MEK on phosphorylation of Akt and Erk induced by 27OHChol.
Fig. 1
Effects of PI3K and ERK inhibitors on the transcription and surface expression of mDC-markers induced by 27OHChol.
(A) THP-1 cells were treated for 2 h with 10 µM of LY294002 (a PI3K inhibitor) or U0126 (an MEK inhibitor) and incubated with 27OHChol (2.5 µg/ml) for 48 h. Transcript levels of CD80, CD83 and CD88 were assessed by real-time PCR. Data are expressed as the mean±SD (n=3 replicates/group). ***p<0.001 versus control; ###p<0.001 versus 27OHChol. (B) THP-1 cells were treated for 2 h with LY294002 or U0126 (10 µM each) followed by an incubation with 27OHChol (2.5 µg/ml) for 48 h. Cells were immunostained with antibodies against CD80, CD83 and CD88 and analyzed by flow cytometry. Results represent one of three independent experiments.
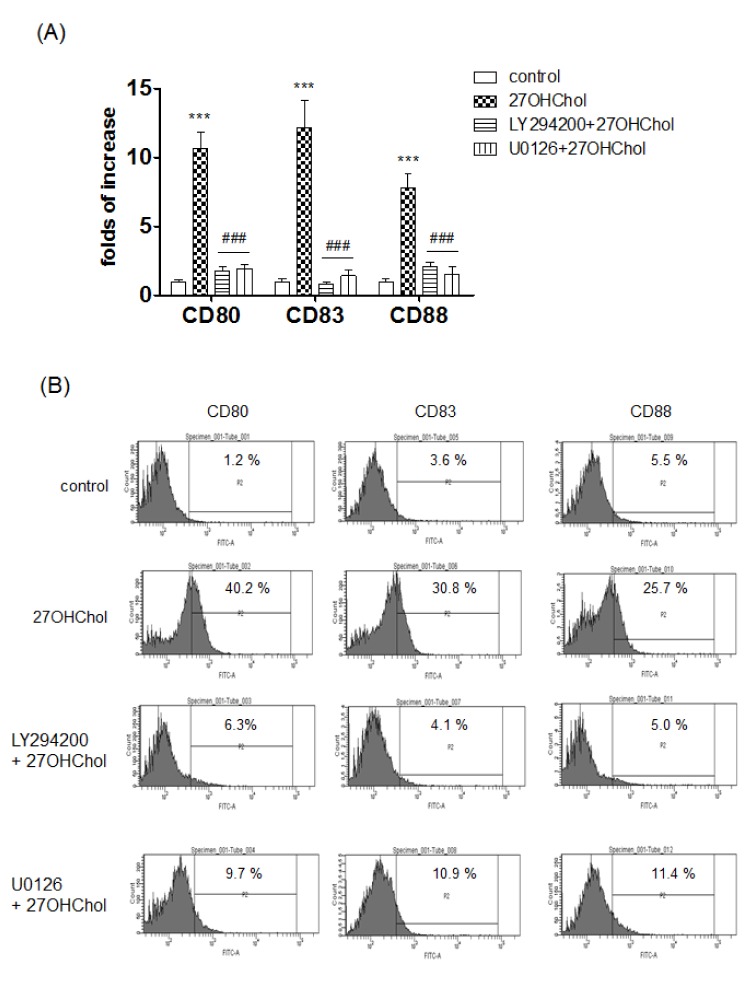
Fig. 2
Effects of inhibition of PI3K and ERK on expression of MHC class I and II molecules induced by 27OHChol.
THP-1 cells were treated with 10 µM of LY294002 (a PI3K inhibitor) or U0126 (an MEK inhibitor) for 2 h followed by stimulation with 27OHChol (2.5 µg/ml) for 48 h. The stimulated cells were immunostained for MHC class I and II. Fluorescence was analyzed by flow cytometry. Results represent one of three independent experiments.
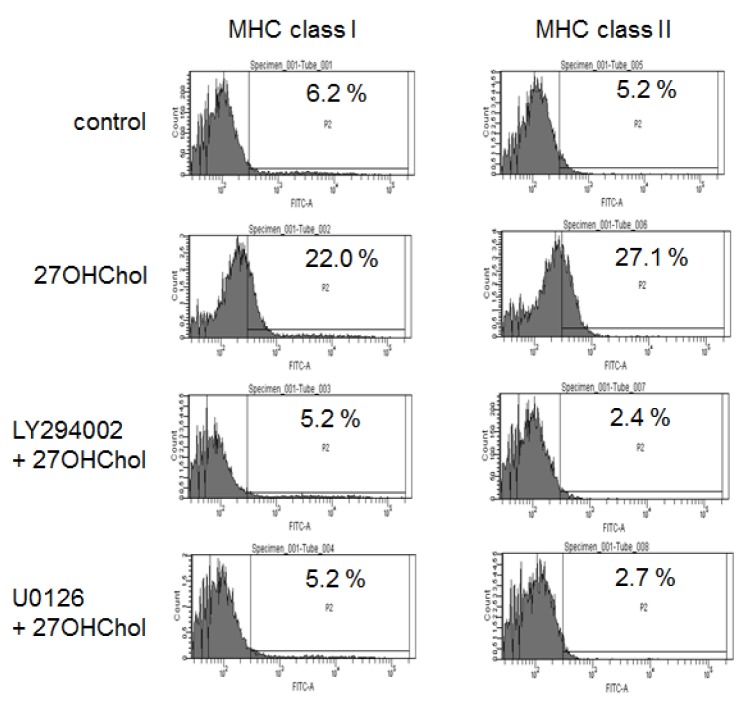
Fig. 3
Effects of inhibition of PI3K and ERK on functional alteration of monocytic cells induced by 27OHChol.
THP-1 cells were treated for 2 h with 10 µM of LY294002 (a PI3K inhibitor) or U0126 (an MEK inhibitor) and stimulated with 27OHChol (2.5 µg/ml) for 48 h. Cells were analyzed by flow cytometry after incubation with 1 mg/ml of FITC-conjugated dextran for 1 h. Results represent one of three independent experiments.
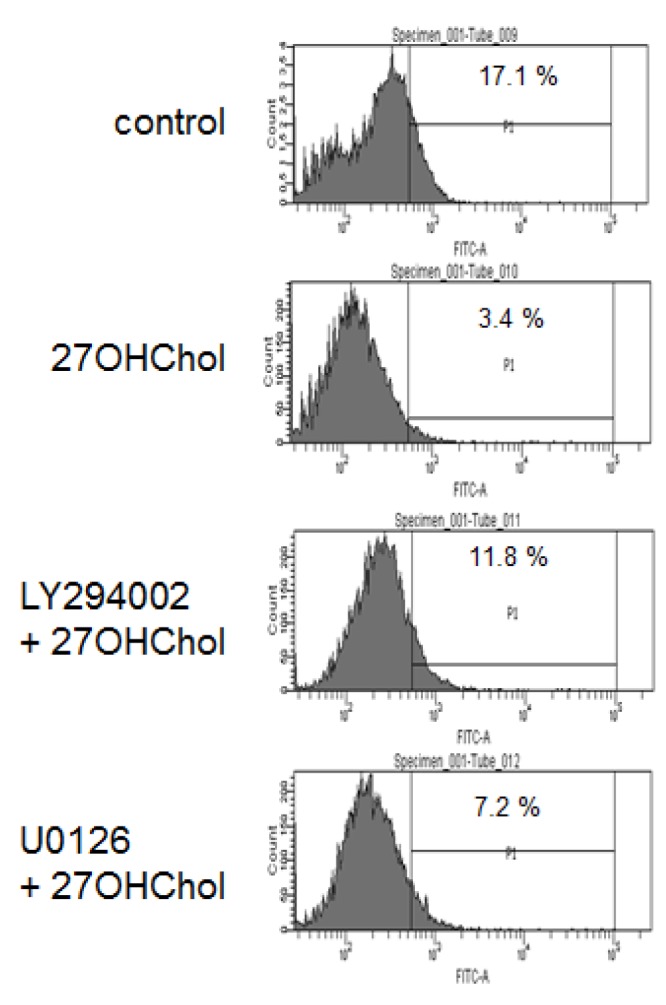
Fig. 4
Effects of PI3K and ERK inhibitors on cell adhesion and expression of CD197.
(A) THP-1 cells were treated for 2 h with 10 µM of LY294002 (a PI3K inhibitor) or U0126 (an MEK inhibitor) and stimulated with 27OHChol (2.5 µg/ml) for 48 h. After harvesting, cells were immunostained with an anti-CD197 antibody and analyzed by flow cytometry. Results represent one of three independent experiments. (B) THP-1 cells were treated for 2 h with LY294002 or U0126 (10 µM each) followed by stimulation with 27OHChol (2.5 µg/ml) for 48 h. After removal of non-adherent cells, adherent cells were counted. Data are expressed as the mean±SD (n=3 replicates/group). ***p<0.001 versus control; ###p<0.001 versus 27OHChol.
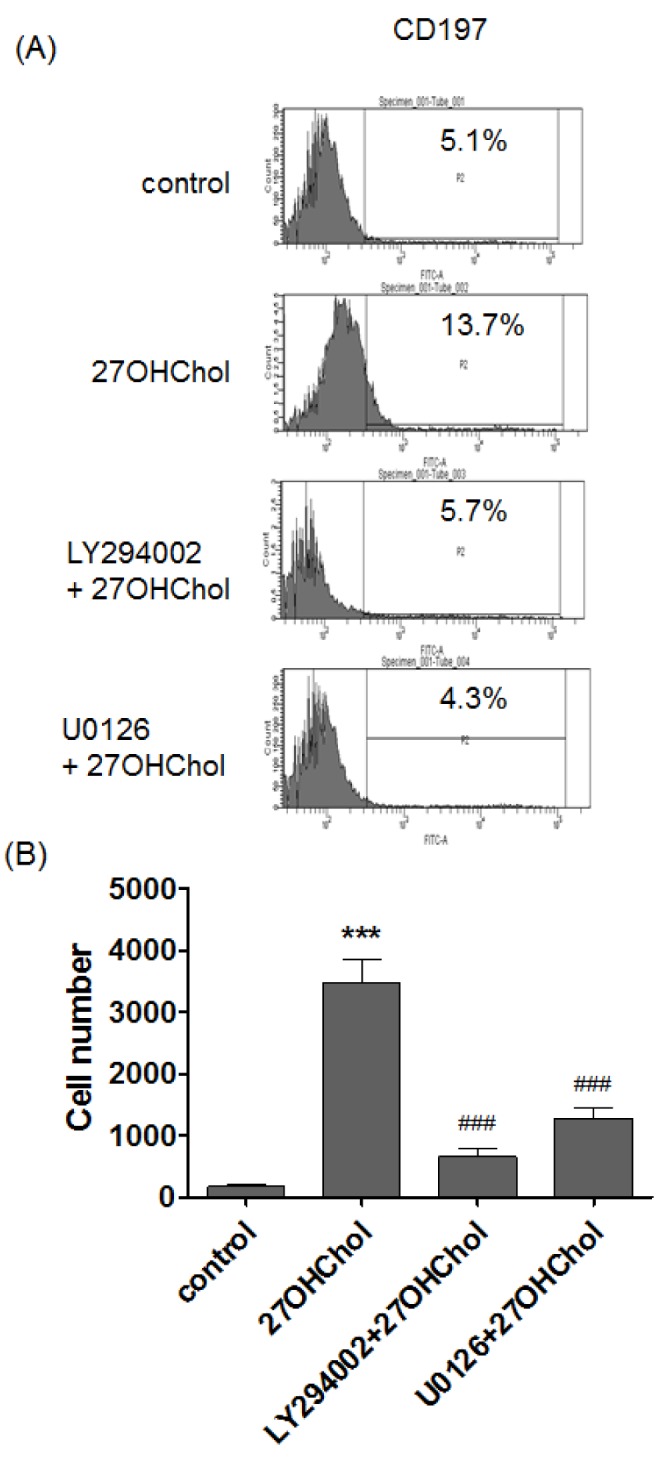
Fig. 5
Effects of PI3K and ERK inhibitors on expression of atherosclerosis-associated CD molecules.
(A) THP-1 cells were treated for 2 h with 10 µM of LY294002 (a PI3K inhibitor) or U0126 (an ERK inhibitor) and stimulated with 27OHChol (2.5 µg/ml) for 48 h. Total RNA was extracted from the cells, and transcript levels of CD105, CD137 and CD166 were assessed by real-time PCR. Data are expressed as the mean±SD (n=3 replicates/group). ***p<0.001 versus control; ###p<0.001 versus 27OHChol. (B) THP-1 cells were treated for 2 h with LY294002 or U0126 (10 µM each) followed by stimulation with 27OHChol (2.5 µg/ml) for 48 h. Cells were immunostained with antibodies against CD105, CD137 and CD166 and analyzed by flow cytometry. Results represent one of three independent experiments.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download