INTRODUCTION
Within minutes of vaginal deposition, sperm begin to leave the seminal fluid and swim toward the ovum. The journey of sperm towards the oocyte is a guided event that is capacitated by changes in the local environment surrounding the sperm, including alkalinization and chemotactic hormonal and temperature gradients, among others. Ion channels in sperm are responsible for capacitation and capacitation-associated activities that mediate the sperm hyperactivation underlying male fertility.
Catsper (cation channel sperm-associated 1) and mSlo3 (mouse slowpoke homologue 3; also known as Kcnu1 [potassium channel subfamily U member 1] and Kcnma3 [potassium Ca2+-activated channel subfamily M alpha 1]) in mice, and HVCN1 (hydrogen voltage-gated channel 1) in humans, are ion channels localized to the principal piece of sperm flagellum that are involved in regulating the sperm hyperactivation process necessary for motility. Whereas the Ca2+ permeating channel Catsper can be activated directly by intracellular alkalinization and progesterone, mSlo3, which mediates a weakly outwardly rectifying K+ conductance in sperm known as KSper, can sense pH or Ca2+ and set the membrane potential in a way that favors Catsper-mediated Ca2+ influx. However, the characteristics of this K+ conductance in human sperm have not been fully elucidated.
Electrophysiological studies have characterized the pH-sensitive K+ conductance that sets the membrane potential in mouse sperm [
123]. Subsequent studies have shown that this current is almost certainly mediated by a complex comprising the pore-forming mSlo3 α-subunit and the auxiliary γ2-subunit, LRRC52 (leucinerich-repeat–containing 52) [
4]. However, the electrophysiological properties of human KSper differ from those of mouse KSper in that KSper can be inhibited by progesterone, exhibits a distinct pharmacological profile, and is more dependent on regulation by Ca
2+ [
5]. On the basis of these observations, Mannowetz et al. proposed that Slo1, also known as the BK
Ca (large-conductance Ca
2+-activated potassium) channel (KCNMA1), rather than Slo3, is responsible for human KSper. Brenker et al. subsequently demonstrated that human KSper exhibits hallmarks of human Slo3 (hSlo3), including insensitivity to the BK
Ca channel inhibitors iberiotoxin (IbTx) and tetraethylammonium (TEA), Ca
2+-dependent rather than pH-dependent activation, and inhibition by progesterone.
Collectively, these studies suggest that discrepancies in physiology between human and mouse spermatozoa may reflect the involvement of a different regulatory subunit or subfamily in the modulation of KSper, highlighting the importance of understanding the specific pharmacological profile of hSlo3.
The fact that mSlo3 is responsible for capacitation in murine sperm has motivated the search for strong pharmacological modulators of hSlo3 that may provide a biological tool for validating the role of hSlo3 in human sperm physiology and help to establish new therapeutic concepts for male infertility. Several recent studies have demonstrated that general BK
Ca channel blockers inhibit heterologously expressed Slo3 and human native KSper. However, the resulting modulators display limited preference for hSlo3 relative to hSlo1, and thus do not markedly discriminate between the two channels
in vivo or
in vitro [
56]. Therefore, we undertook an alternative approach, applying well-known BK
Ca activators to heterologously expressed hSlo3 K
+ channels and validating their electrophysiological properties. We found that the BK
Ca activators, LDD175 (which is recognized also as CTBIC) and NS1619, inversely regulated hSlo3, with NS1619 suppressing hSlo3 activity and LDD175 enhancing hSlo3 activity in a Ca
2+-dependent manner, rather than an intracellular pH (pH
i)-dependent manner.
Go to :

DISCUSSION
Sperm-specific ion channels contribute to the changes in membrane potential, intracellular Ca2+ and pHi that control not only the acrosomal reaction, but also sperm motility. However, the molecular identity of the membrane potential-regulating KSper in human sperm is still controversial. Thus, studying the pharmacological properties of this current is beneficial for understanding the molecular nature of the sperm K+ channel.
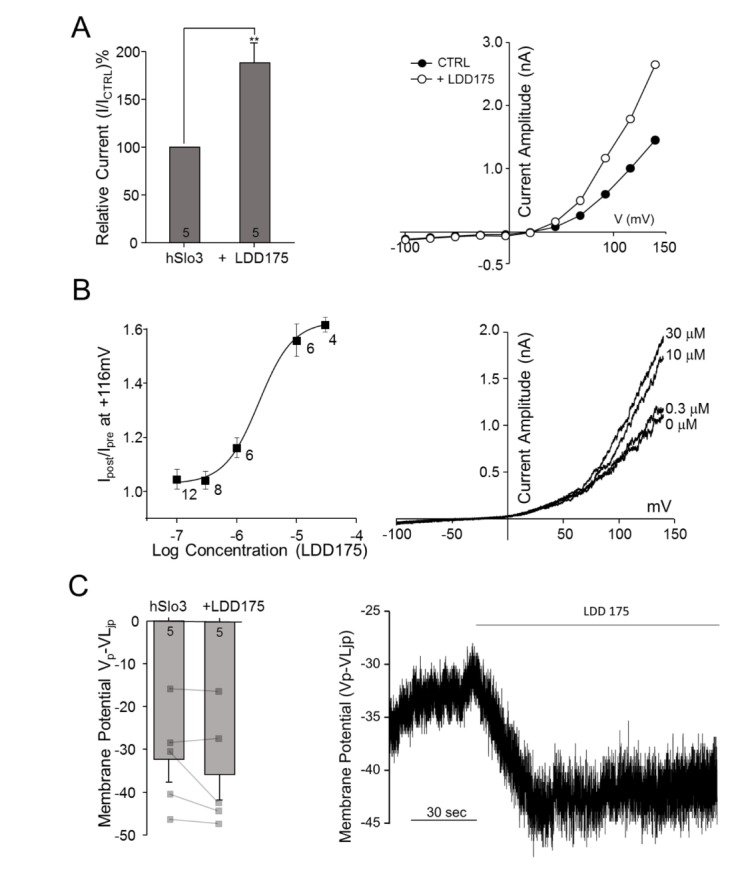
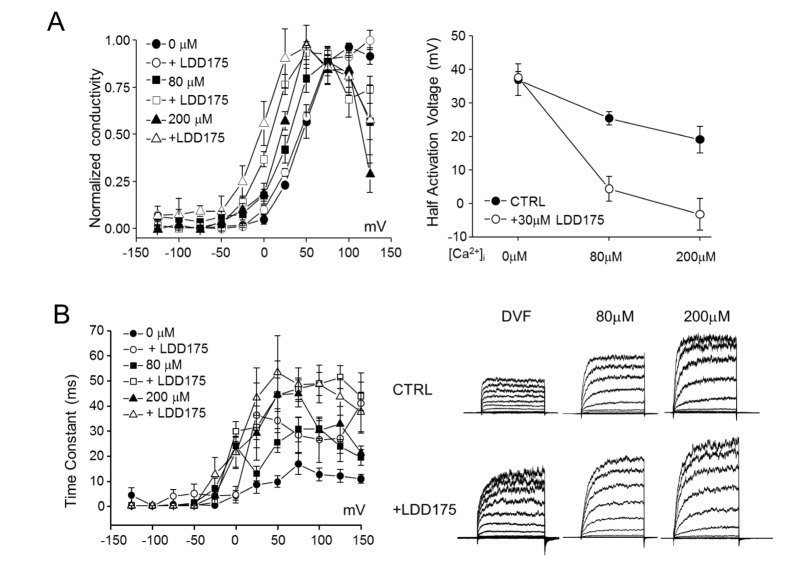
Here, we show for the first time that two general activators of BKCa channels differentially regulate sperm-specific hSlo3 channel, a molecular candidate for human KSper. Interestingly, the well-known BKCa channel opener, NS1619, inhibited the K+ current mediated by heterologously expressed hSlo3+hLRRC52 in a concentration-dependent manner. By contrast, LDD175, another novel BK channel activator, potentiated the hSlo3 K+ current in a concentration-dependent manner. Moreover, whereas LDD175 negatively shifted the G~V relationship of rSlo1, regulating it over a physiological range of membrane potentials, as is well known; an equivalent concentration of LDD175 did not alter the half-maximal voltage for activation of hSlo3 in the absence of intracellular Ca2+, regardless of pHi. However, LDD175-induced activation of hSlo3 was enhanced in the presence of intracellular Ca2+, which shifted its current-voltage relationship to more negative voltages.
Genetic ablation of Slo3 has revealed that mSlo3, a pH-sensitive, Ca
2+-independent channel, is the principal K
+ channel in murine sperm. Moreover, its γ2 axillary subunit, LRRC52, affects the activation voltage of mSlo3, causing the channel to gate at physiological membrane potentials [
478]. Unexpectedly, the K
+ current recorded from human sperm exhibited clear differences in pharmacological properties, including pH
i-dependence and intracellular Ca
2+ sensitivity. Specifically, whereas mouse KSper is only sensitive to internal alkalinization, human KSper activates in an intracellular Ca
2+-dependent manner with minimal regulation by pHi [
9]. These differences between mouse and human KSper inform the debate surrounding the molecular identity of human KSper.
It is known that stimuli including alkalinization and progesterone can cause intracellular Ca
2+ to rise in human sperm, leading to hyperactivated motility, capacitation, and the acrosome reaction [
10]. Although the precise mechanism is not completely understood, Ca
2+ channels and K
+ channels play a role in controlling sperm membrane potential and [Ca
2+]
i. In particular, pH-sensitive efflux of K+ is detected in sperm and is thought to aid in hyperpolarization of the membrane potential, which would maximize Ca
2+ entry through CatSper channels. Therefore, understanding the properties of human KSper would provide important insight into the physiology of sperm.
It is known that a broad range of BK
Ca channel blockers, including quinidine, barium and TEA, exert inhibitory action on either native human KSper or hSlo3 currents in heterologous overexpression systems [
11]. However, we found that IbTx, a selective BK
Ca blocker, and 4-AP, a voltage-gated K
+ channel blocker, had minimal effects on hSlo3 currents in transfected HEK293 cells. We also found that 4-AP had a modest potentiation at the -50 mV to +80 mV while there is no significant difference on peak amplitude. 4-AP was known to inhibit mouse Slo3 intracellularly while it did not exhibit the inhibitory effect extracellularly [
11]. Mansell et al. showed that sustained outward KSper current from human sperm was ineffective, instead, transient tail current of KSper was potentiated by 4-AP as well as progesterone [
12]. Therefore, the potentiation of hSlo3 in the range of depolarized membrane potential is evidence of the functional role of Slo3 in KSper.
Mannowetz et al. reported that Slo1 is the principal channel underlying KSper in human sperm, based on their observation that the peptide toxins, charybdotoxin and IbTx, as well as progesterone, inhibited this current; by contrast, mouse KSper was found to be insensitive to these agents [
5]. However, Brenker et al. subsequently refuted this idea, demonstrating a different pharmacological profile that human KSper was insensitive to IbTx and progesterone could not elicit Slo1 [
6]. Our data support the conclusion that hSlo3 is insensitive to IbTx and 4-AP, and is minimally regulated by changes in pHi. However, these observations are contrast to the previously reported pHi-sensitivity of human KSper [
612]. These discrepancies may reflect the possibility that other functional subunits of Ca
2+-sensitive K
+ channels, including β- or γ-subunits, modify access of toxins and blockers to their binding sites on pore-forming α-subunits. It is also possible that differences in extracellular Ca
2+, which can inhibit monovalent CatSper currents (as Brenker et al. have argued) may contribute to reported differences in KSper. Taken together, our pharmacological data suggest that the human α-subunit, Slo3, and its axillary γ2-subunit, LRRC52, underlie KSper and play an important role in human sperm physiology.
Several activators of BK
Ca channels that act through a variety of mechanisms have been developed and used extensively for studying the function of BK
Ca channels in biological systems. Among them, NS1619, a synthetic benzimidazolone derivative, activates the BK
Ca channel either through direct interactions with the channel [
13] or through the closely associated release of internal Ca
2+ [
14]. It has also been reported that NS1619 activates Slo1 by functionally interacting with the S6/RCK linker connecting the transmembrane S6 segment with the cytosolic RCK1 domain [
15]. However, none of these studies evaluated changes in Slo3 activity caused by NS1619, which our data show caused a concentration-dependent inhibition of hSlo3 K
+ current. This inhibitory action of NS1619 may interfere with the ability of α (Slo3) and γ2 subunits to potentiate channel activity; alternatively, the binding regions in the S6/RCK linker of Slo3 that promote the open state of the channel may differ from those in Slo1.
LDD175, a synthetic benzofuroindole analogue, activates the BK
Ca channel through specific effects on the α-subunit. Since Gormemis et al. first reported the potent activity of the BK
Ca channel expressed in
Xenopus oocytes [
16], LDD175 has demonstrated relaxation-inducing actions on the urinary bladder, antispasmodic actions on intestinal motility, and erectile responses in corporal smooth muscle [
171819]. Although the underlying mechanism of action of LDD175 on BK
Ca is not fully elucidated, it is known that LDD175 shifts the conductance-voltage relationship of the channel leftward without changing its voltage dependence [
20]. It is also known that LDD175 acts extracellularly at the interface between the turret region and the P-region of the Slo1 α-subunit to potentiate BK
Ca channel activity [
21]. In addition, the fact that the presence of β-subunits has only a minor effect on LDD175 actions implies that the interaction occurs only in the α-subunit.
Our data show that 10 µM LDD175 dramatically shifted the half-activation voltage of rSlo1 to a hyperpolarized potential, whereas this concentration of LDD175 had no significant effect on hSlo3 at pH 7.3 or pH 8.0 in the absence of Ca2+. However, LDD175 caused a shift in the half-activation voltage in the presence of intracellular Ca2+ similar to that of rSlo1.
A sequence alignment reveals four amino acids of hSlo3 that are positioned at sites homologous to those in rSlo1 proposed to be critical for LDD175 binding to the rSlo1 α-subunit [
21]. Therefore, the mechanism of action of LDD175 on hSlo3 may be similar to that on rSlo1, where it interacts extracellularly at the interface between a-subunits.
Although our data demonstrate that the activity of hSlo3 is dependent on intracellular Ca2+, this isoform lacks negatively charged amino acids that account for intracellular Ca2+ binding in Slo1. Further investigations should seek to elucidate the mechanism underlying the Ca2+-dependent regulation of Slo3, which is important in sperm physiology.
In summary, the present results have defined distinct pharmacological differences between the closely related Slo1 and Slo3 K+ channels using two activators with different mechanisms of action. To date, hSlo3, a strong molecular candidate for KSper, exhibits LDD175-dependent activation, which may explain how KSper regulates membrane potential and consequentially regulates intracellular Ca2+ increases through CatSper in human sperm. Therefore, further investigation is clearly required to determine whether native KSper shares LDD175-activation and NS1619-inhibition features similar to those of heterologously expressed hSlo3.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download