INTRODUCTION
METHODS
RESULTS
Effects of acidic pH on voltage-gated K+ channels
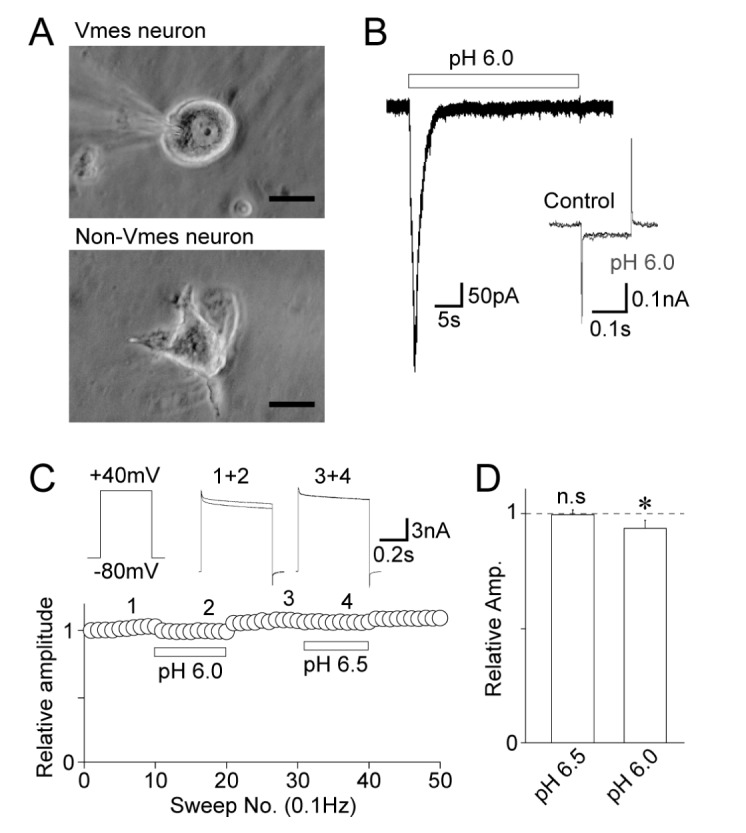 | Fig. 1Effects of acidic pH on voltage-dependent K+ currents.(A) Phase contrast images of Vmes (upper) and non-Vmes (lower) neurons isolated from the Vmes region. While Vmes neurons, which are primary sensory neurons, generally had a round or oval soma without any dendritic processes, non-Vmes neurons, which are central neurons, have several dendritic processes. Scale bars: 20 µm. (B) pH 6.0-induced current recorded from Vmes neurons. Note that the acid-induced currents returned to the basal current levels within 10 s of the application of the pH 6.0 solution. Inset: typical current responses to hyperpolarizing voltage step pulses (–10 mV, 150 ms duration) before (black) and 30 s after the application of the pH 6.0 solution (gray). (C) A typical time course of the peak amplitude of voltage-dependent K+ currents before, during, and after the application of a solution with an acidic pH. Insets represent raw traces of voltage-dependent K+ currents at numbered regions. (D) Acidic pH-induced changes in the peak amplitude of voltage-dependent K+ currents. Each column represents the mean and standard error of mean from 8 and 9 experiments for the pH 6.5 and pH 6.0 conditions, respectively. *p<0.05; n.s: not significant.
|
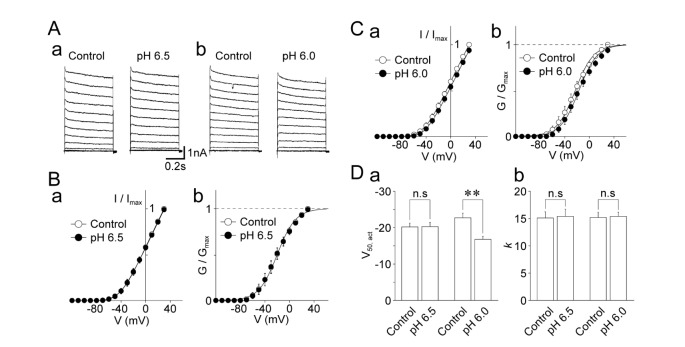 | Fig. 2Effects of acidic pH on the voltage-dependence of voltage-dependent K+ currents.(A) Typical traces of voltage-dependent K+ currents before (left) and during (right) the application of a solution with an acidic pH (a: pH 6.5; b: pH 6.0). (B) Current-voltage (a) and conductance-voltage (b) relationships of voltage-dependent K+ channels in the control (open circles) and pH 6.5 conditions (closed circles). Each circle and error bar represents the mean and standard error of mean (SEM) from 8 experiments. (C) Current-voltage (a) and conductance-voltage (b) relationships of voltage-dependent K+ channels in the control (open circles) and pH 6.0 conditions (closed circles). Each circle and error bar represents the mean and SEM from 10 experiments. (D) Acidic pH-induced changes in V50,act (a) and k (b) of voltage-dependent K+ channels. Each column and error bar represents the mean and SEM from 8 and 10 experiments for the pH 6.5 and pH 6.0 conditions, respectively. **p<0.01; n.s: not significant.
|
Effects of acidic pH on HCN channels
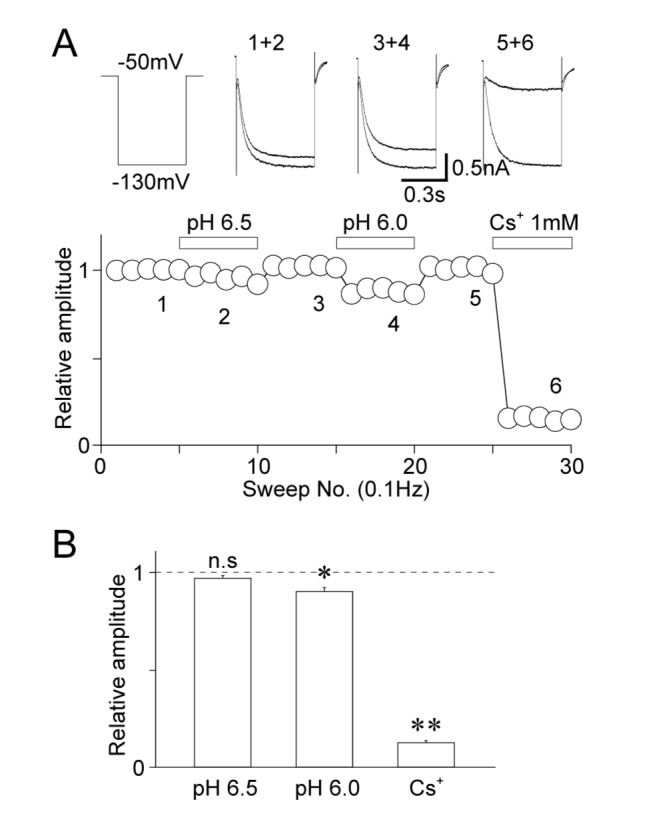 | Fig. 3Effects of acidic pH on cyclic nucleotide-activated cation (HCN) currents.(A) A typical time course of the peak amplitude of HCN currents before, during, and after the application of a solution with an acidic pH and 1 mM Cs+. Note that Cs+, a general HCN channel blocker, greatly inhibited the membrane currents induced by hyperpolarizing voltage stimuli. Insets represent raw traces of HCN currents at numbered regions. (B) Acidic pH-induced changes in the peak amplitudes of HCN currents. Each column represents the mean and standard error of mean from 9, 9, and 6 experiments for the pH 6.5, pH 6.0, and Cs+ conditions, respectively. *p<0.05, **p<0.01; n.s: not significant.
|
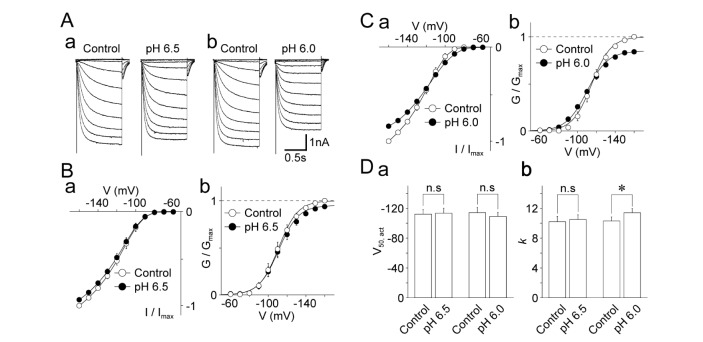 | Fig. 4Effects of acidic pH on the voltage-dependence of cyclic nucleotide-activated cation (HCN) currents.(A) Typical traces of HCN currents before (left) and during (right) the application of a solution with an acidic pH (a: pH 6.5; b: pH 6.0). (B) Current-voltage (a) and conductance-voltage (b) relationships of HCN channels in the control (open circles) and pH 6.5 conditions (closed circles). Each circle and error bar represents the mean and standard error of mean (SEM) from 9 experiments. (C) Current-voltage (a) and conductance-voltage (b) relationships of HCN channels in the control (open circles) and pH 6.0 conditions (closed circles). Each circle and error bar represents the mean and SEM from 10 experiments. (D) Acidic pH-induced changes in V50,act (a) and k (b) of HCN channels. Each column and error bar represents the mean and SEM from 9 and 10 experiments for the pH 6.5 and pH 6.0 conditions, respectively. *p<0.05; n.s: not significant.
|
Effects of acidic pH on voltage-gated Ca2+ channels
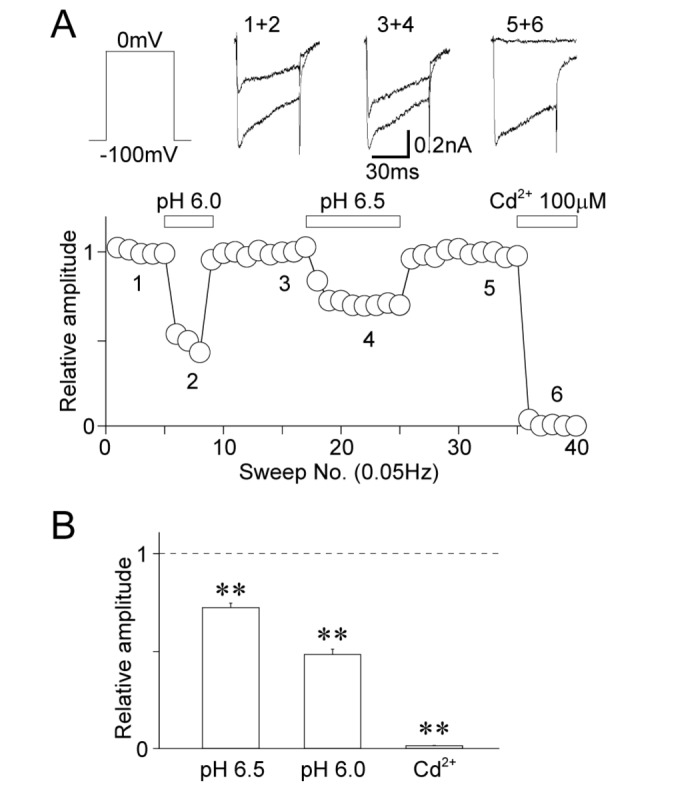 | Fig. 5Effects of acidic pH on voltage-dependent Ca2+ currents.(A) A typical time course of the peak amplitude of voltage-dependent Ca2+ currents before, during, and after the application of a solution with an acidic pH and 100 µM Cd2+. Note that Cd2+, a general voltage-dependent Ca2+ channel blocker, greatly inhibited the membrane currents induced by depolarizing voltage stimuli. Insets represent raw traces of voltage-dependent Ca2+ currents at numbered regions. (B) Acidic pH-induced changes in the peak amplitude of voltage-dependent Ca2+ currents. Each column represents the mean and standard error of mean from 8, 9, and 9 experiments for the pH 6.5, pH 6.0, and Cd2+ conditions, respectively. **p<0.01.
|
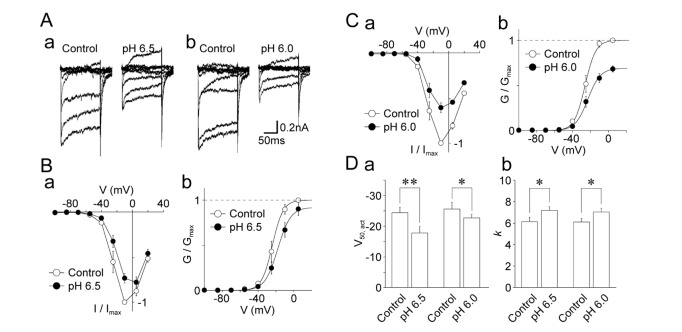 | Fig. 6Effects of acidic pH on the voltage-dependence of voltage-dependent Ca2+ currents.(A) Typical traces of voltage-dependent Ca2+ currents before (left) and during (right) the application of a solution with an acidic pH (a: pH 6.5; b: pH 6.0). (B) Current-voltage (a) and conductance-voltage (b) relationships of voltage-dependent Ca2+ channels in the control (open circles) and pH 6.5 conditions (closed circles). Each circle and error bar represents the mean and standard error of mean (SEM) from 8 experiments. (C) Current-voltage (a) and conductance-voltage (b) relationships of voltage-dependent Ca2+ channels in the control (open circles) and pH 6.0 conditions (closed circles). Each circle and error bar represents the mean and SEM from 10 experiments. (D) Acidic pH-induced changes in V50,act (a) and k (b) of voltage-dependent Ca2+ channels. Each column and error bar represents the mean and SEM from 8 and 10 experiments for the pH 6.5 and pH 6.0 conditions, respectively. *p<0.05, **p<0.01.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download