INTRODUCTION
METHODS
Cell lines
Methyl thiazolyl tetrazolium (MTT) assay
Lactate dehydrogenase (LDH) assay
Morphologic assay
Scratch wound healing assay
Statistical analysis
RESULTS
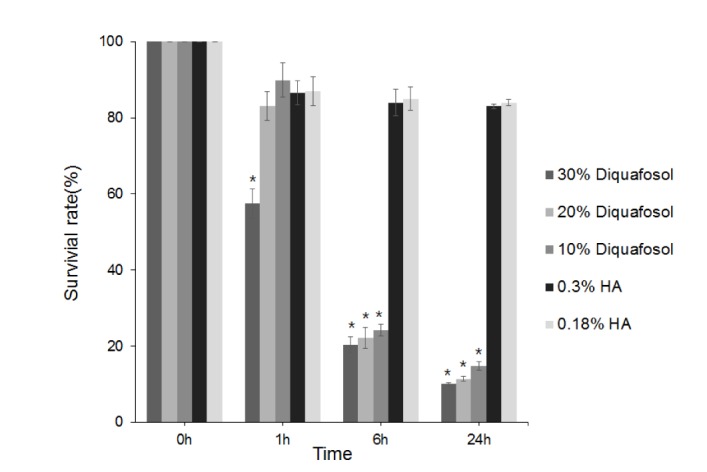 | Fig. 1Metabolic activities of human corneal epithelial cells as determined by methyl thiazolyl tetrazolium (MTT) assay after 1, 6, or 24 hours of treatment with 3% diquafosol, or 0.3% or 0.18% hyaluronic acid (HA).Cell viability significantly decreased after treatment for 1 hour with 30% diluted diquafosol and after 6 hours of treatment with 10% or 20% diluted diquafosol. The two HAs did not significantly change cell viability. Survival rates are provided as mean±SEMs and *means significantly different (p<0.05).
|
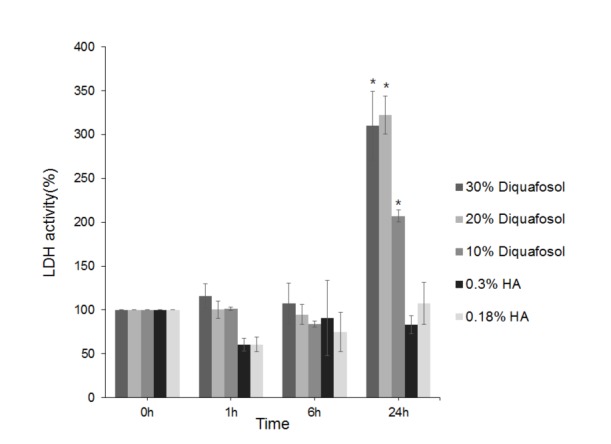 | Fig. 2Lactate dehydrogenase (LDH) activities of human corneal epithelial cells after treatment with 3% diquafosol or 0.3% or 0.18% hyaluronic acid (HA) for 1, 6, or 24 hours.Diquafosol increased LDH activities all times versus HAs, and significantly increased LDH activity after 24 hours. Survival rates are presented as means±SEMs and * means significantly different (p<0.05).
|
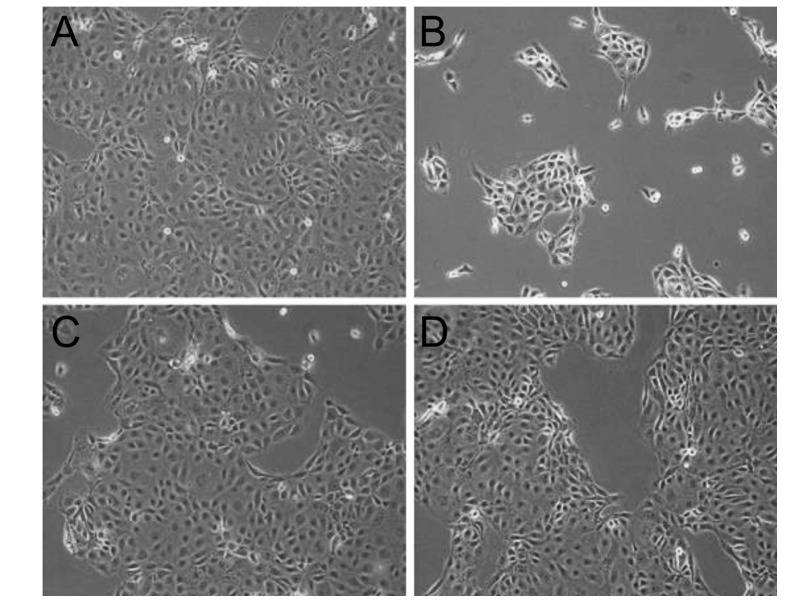 | Fig. 3Inverted phase-contrast micrographs of human corneal epithelial cells exposed to 0.3% diquafosol or 0.3% or 0.18% hyaluronic acid (HA) (original magnification, ×200).Many epithelial cells were visible in control culture media (A). HCECs were more detached from dishes after treatment with 0.3% diquafosol (B) than after treatment with 0.3% (C) or 0.18% (D) HA.
|
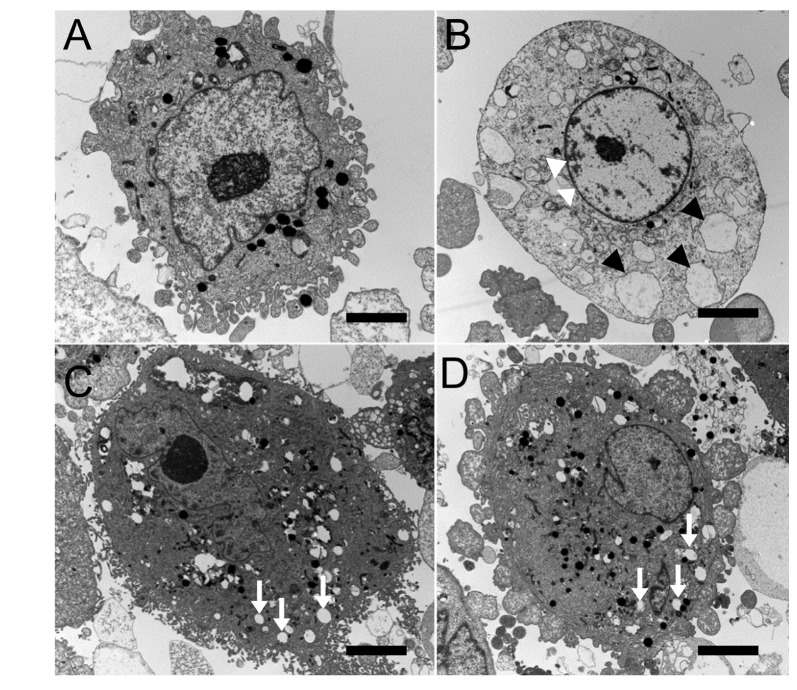 | Fig. 4Transmission electron micrographs of human corneal epithelial cells exposed to 0.3% diquafosol, or 0.3% or 0.1% hyaluronic acid (HA) (bar length=2 mm: original magnification, ×3000~4000).Normal corneal epithelial cells (A) showing microvilli, homogenous cytoplasm, and intact cell and nuclear membranes. Cells treated with diquafosol (B) showing loss of microvilli, condensed nuclear remnant along nuclear peripheries (white arrowheads), and cytoplasmic vacuole formation presumed to be due to swollen cytoplasmic organelles (black arrowheads). When exposed to 0.3% (C) or 0.18% (D) HA, corneal epithelial cells appeared normal with the exception of well-developed cytoplasmic vacuoles (white arrows).
|
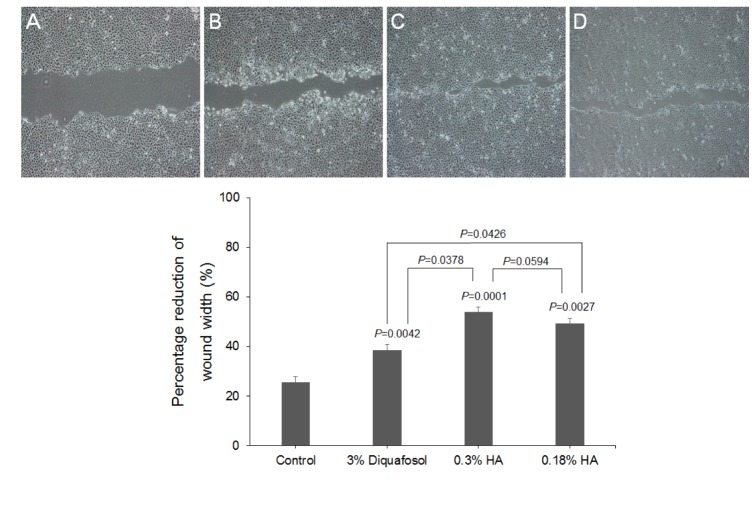 | Fig. 5The closure of HCEC wounds in response to 3% diquafosol, or 0.3% or 0.18% hyaluronic acid (HA).The effects of diquafosol and HAs on wound closure are expressed as percentage reductions in average wound width 24 hours after confluent HCECs were scratched in the absence (A) or presence of diquafosol (B) or HAs (C&D) (2 mg/mL). Results are the means±SDs (n=3) of percentage reductions in wound widths, which were determined by measuring average widths at 10 positions. HAs and diquafosol were found to significantly stimulate wound closure versus control, and HAs had greater effects than diquafosol.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download