INTRODUCTION
METHODS
Mice
RF-EMF exposure system
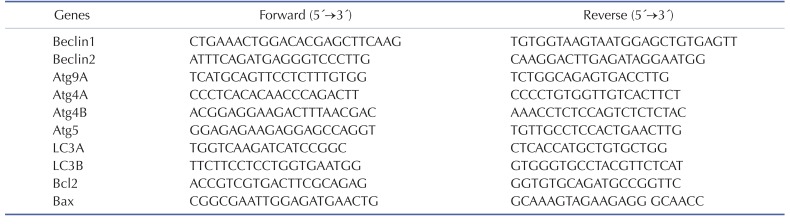
Quantitative real-time PCR and semi-quantitative RT-PCR
Western blotting analysis
Transmission electron microscopy (TEM)
Statistical analysis
RESULTS
Autophagy related genes significantly increased in the cerebral cortex but not in the brainstem
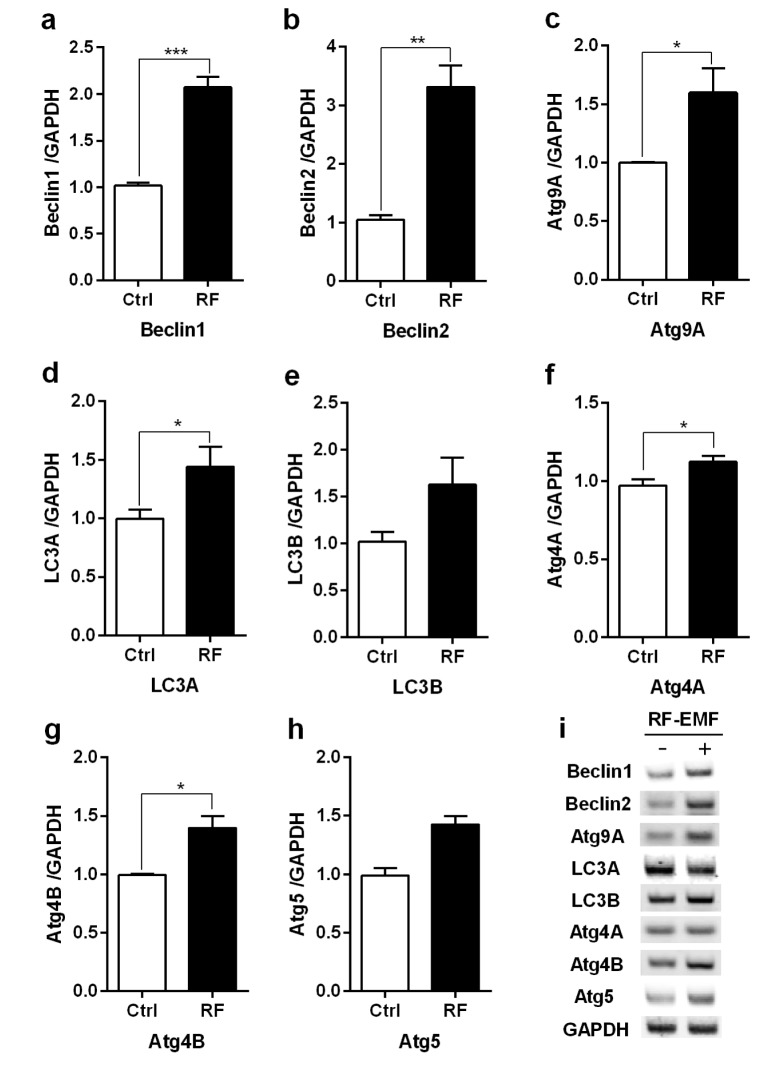 | Fig. 1The transcription levels of autophagic-related genes in the cerebral cortex of mice in response to RF-EMF exposure for 4 weeks.Total RNA was extracted from the cerebral cortex of sham-exposed and RF-EMF exposed mice and were analysed by quantitative real-time PCR to determine the expression level of autophagy genes. (a~h) Quantification of Atg4A/B, Beclin1/2, Atg5, Atg9A, LC3A/B mRNA transcripts by qRT-PCR. (i) 1.5% Agarose gel electrophoresis indicating the differential expression of autophagy genes by sqRT-PCR. The expressional values of the cerebral cortex of the RF-EMF exposed mice were normalized to those of the sham-exposed mice. The relative transcriptional levels of each gene were calculated by normalizing to the expression of GAPDH using the 2−ΔΔCt method (n=5). Each bar represents the mean±SEM of three independent experiments. Statistical significance was evaluated using a t-test: *p<0.05, **p<0.01, ***p<0.001.
|
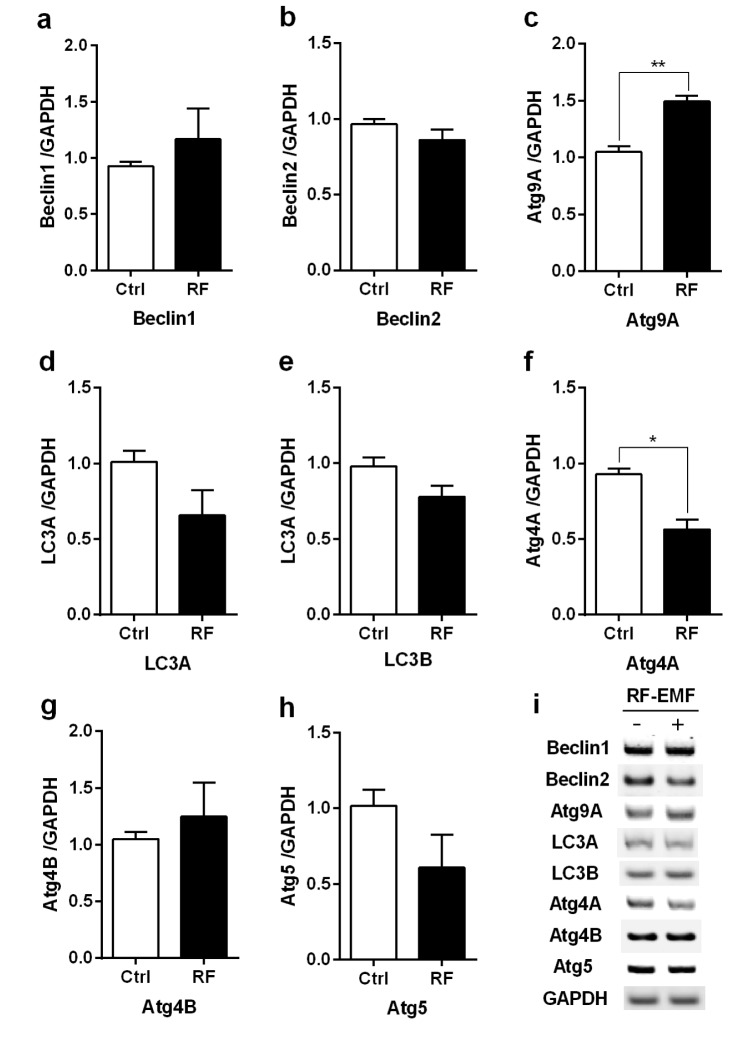 | Fig. 2The transcription levels of autophagic-related genes in the brainstem of mice following 4 weeks of exposure to RF-EMF signals.Total RNA was extracted from the brainstem of sham-exposed and RF-EMF exposed mice and were analysed for the expressional levels of autophagy genes by quantitative real-time PCR. (a~h) Quantification of Atg4A/B, Beclin1/2, Atg5, Atg9A, LC3A/B mRNA transcripts by qRT-PCR. (i) 1.5% Agarose gel electrophoresis showing differential expression of autophagy genes by sqRT-PCR. The expressional values of the cerebral cortex of RF-exposed mice were normalized to those of the sham-exposed mice. The relative transcriptional levels of each gene were calculated by normalizing to the expression of GAPDH using the 2−ΔΔCt method (n=5). Each bar shows the mean of three independent experiments with SEM. Statistical significance was evaluated using a t-test: *p<0.05, **p<0.01.
|
LC3B-II and Beclin1 protein is significantly upregulated in the cerebral cortex but not in the brainstem
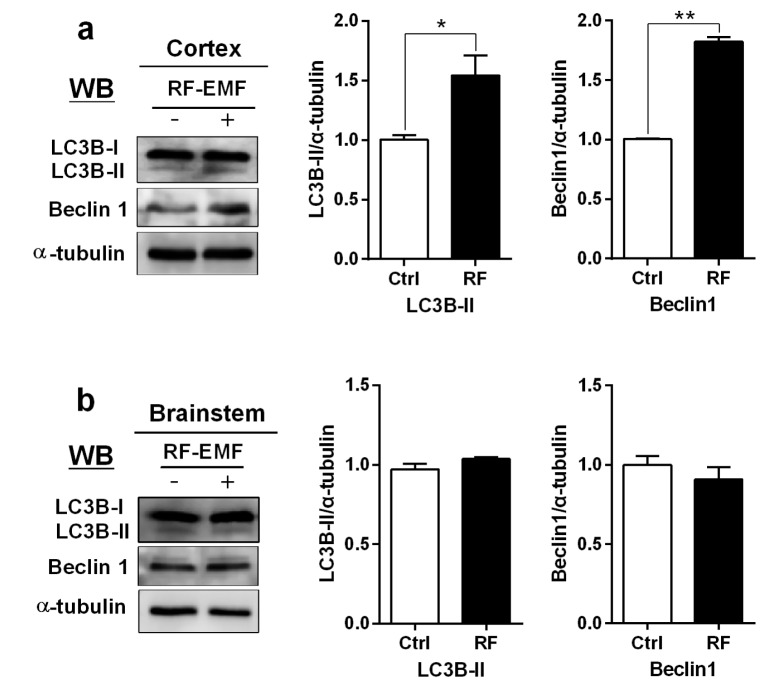 | Fig. 3Expression level for LC3B-II and Beclin1 proteins in the cerebral cortex and brainstem of mice after 4 weeks of exposure to RF-EMF radiation.(a) Total lysates extracted from the cerebral cortex and brainstem of mice was subjected to 15% SDS–PAGE and western-blotted with antibody against LC3B-II and Beclin1 (Cell Signaling Technology, Beverly, MA, USA). α-tubulin was used as the internal loading control. (b) The band intensity of western blot was quantified by densitometry. The protein level was normalized relative to α-tubulin. Each bar shows the mean of three independent experiments with SEM. Statistical significance was evaluated using two tailed t-test: *p<0.05, **p<0.01.
|
Apoptosis is down-regulated in the cerebral cortex but augmented in the brainstem
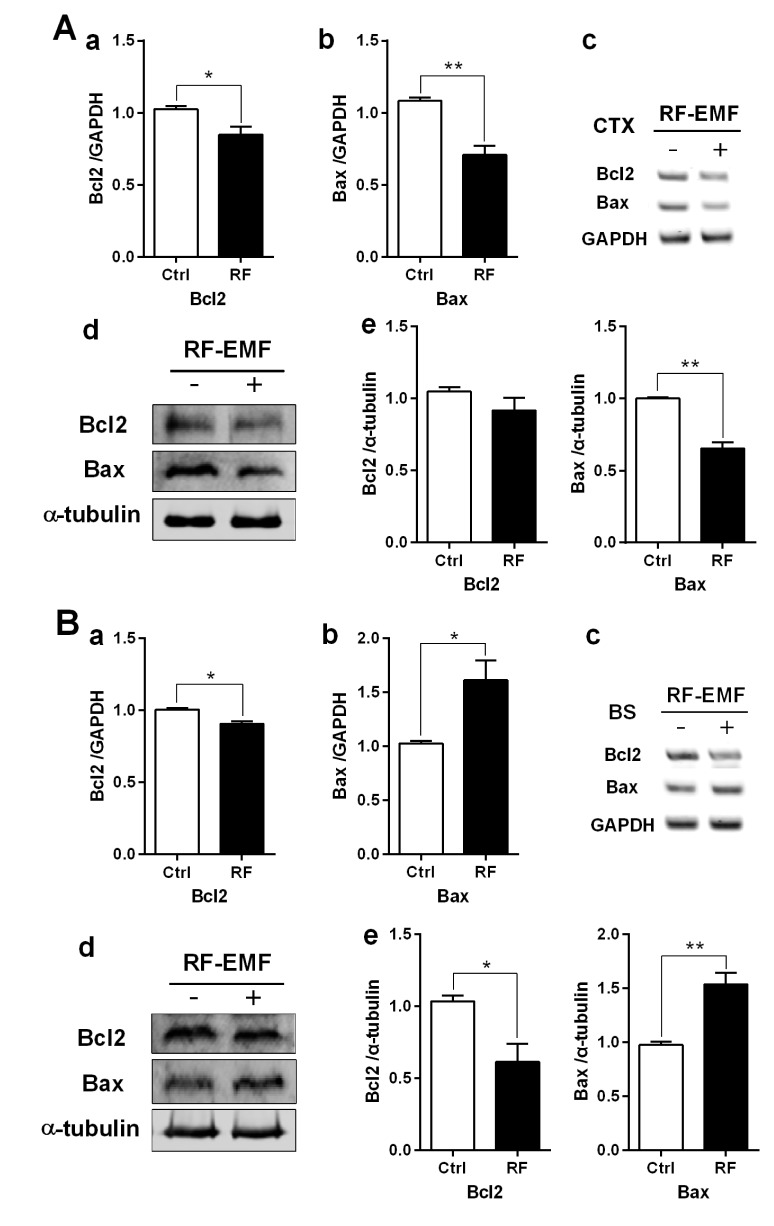 | Fig. 4The expression levels of apoptosis related genes in the cerebral cortex or brainstem of mice following 835 MHz RF-EMF exposure for 4 weeks.The cerebral cortical (A) or brainstem (B) RNA and proteins extracted from sham-exposed and RF-exposed mice were analysed to determine the expression level of apoptotic genes or proteins. (a~b) Quantification of Bcl2 and Bax mRNA transcripts by qRT-PCR. (c) 1.5% Agarose gel electrophoresis showing differential expression of Bcl2 and Bax by sqRT-PCR. The expression values of the cerebral cortex of RF-exposed mice were normalized to those of the sham-exposed mice. The relative mRNA levels of each gene were calculated by normalizing to the expression of GAPDH using the 2−ΔΔCt method (n=5). (d) Total proteins were subjected to 15% SDS–PAGE and immunoblotted with antibodies against Bcl2 and Bax. α-tubulin was used as the loading control. (e) The intensity of western blot bands was quantified by densitometry. The protein level was normalized relative to α-tubulin. Each bar represents the mean±SEM of three independent experiments. Statistical significance was evaluated using a t-test: *p<0.05, **p<0.01.
|
Autophagy is accumulated in the cerebral cortical neuron
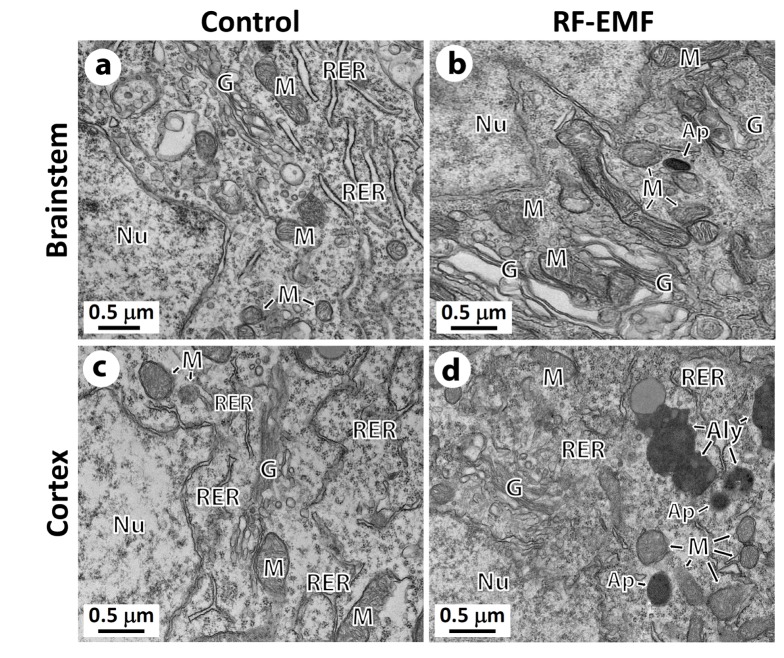 | Fig. 5Representative ultrastructure images showing the autophagic structure in the neuronal cell body of the cerebral cortex or brainstem following 4 weeks of exposure to RF-EMF.Ultrastructural comparison of autophagy between sham control vs RF-EMF exposed group. Representative TEM micrographs were acquired from sham control (a and c) and RF-EMF exposed mice (b and d). Autophagosome (Ap) and autolysosome (Aly) were clearly observed in RF-EMF exposed cortex. Abbreviations are: Ap, autophagosome; Aly, autolysosome; G, Golgi apparatus; M, mitochondria; N, nucleus; Ph, phagophore; RER, rough endoplasmic reticulum. Size bars: 500 nm.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download